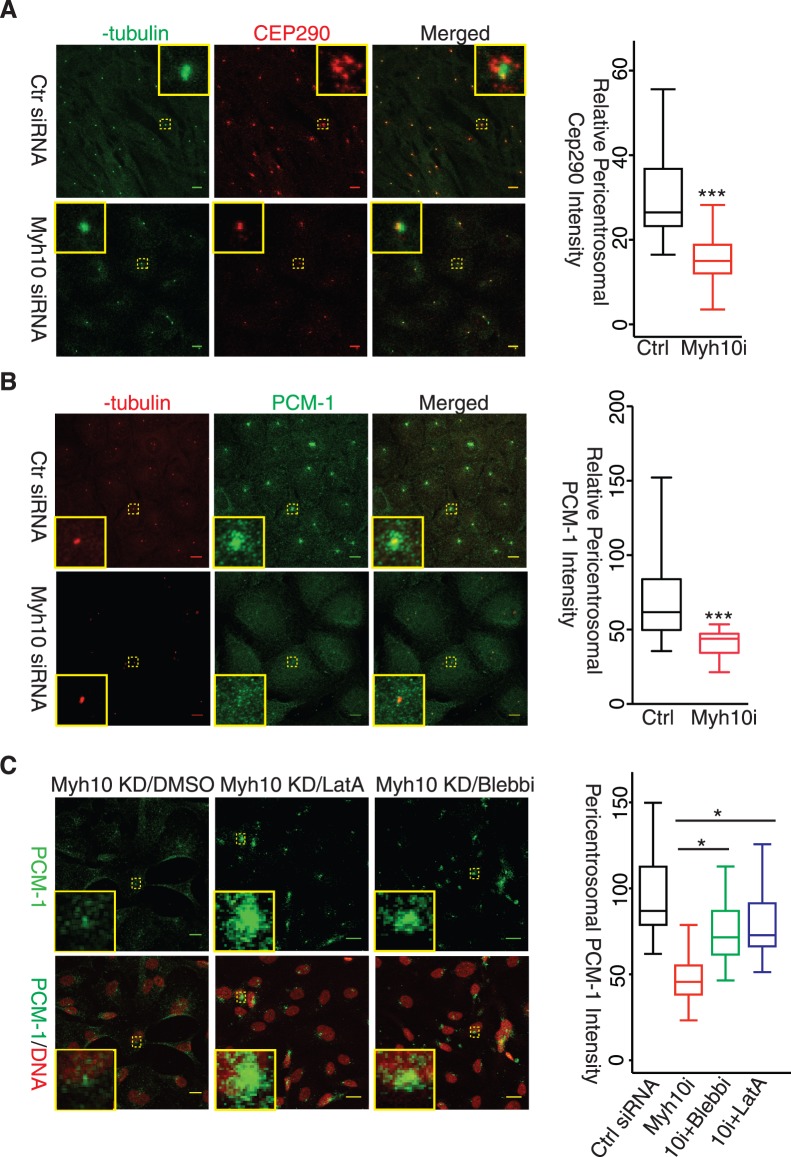
Figure 4. Myh10 Regulates Cep290-Pcm-1 Centriolar Satellites Organization.
(A) Myh10 knockdown prevented Cep290 centriolar satellites formation. RPE-Mchr1GFP cells were transfected with control (left panel, upper row) and Myh10 (left panel, lower row) siRNA and immunostained with γ-tubulin (left panel, green) and Cep290 (left panel, red) antibody. Yellow insets were magnified to allow better visualization of representative images. A 30-pixel diameter circle around the centrosome was drawn for each cell and Cep290 staining intensity within that circle was quantified and plotted (right panel). Scale bars: 10 µm. t-test, p<0.001. (B) Myh10 knockdown reduced Cep290 staining in the pericentrosomal region. Control and Myh10 siRNA transfected RPE-Mchr1GFP cells were fixed and stained for γ-tubulin (red) and PCM-1 (green) (left panel). Pericentrosomal PCM-1 staining was quantified and plotted as described in (A). Yellow insets were magnified to allow better visualization of representative images. Scale bars: 10 µm. t-test, p<0.001. (C) Actin destabilization and Myh9 inhibition restored centriolar satellite PCM-1. Myh10 knockdown RPE-Mchr1GFP cells were treated with DMSO (left panel), 20 nM latrunculin A (middle) and 25 µM blebbistatin for 24 hours then labeled with PCM-1 antibody (green). Yellow boxes show magnified representative images of pericentrosomal PCM-1 cluster. Pericentrosomal PCM-1 cluster intensity was quantified and plotted (right panel). Black, control siRNA with DMSO; red, Myh10 siRNA with DMSO; green, Myh10 siRNA with 25 µM blebbistatin; purple, Myh10 siRNA with 20 nM latrunculin A. *, t-test p<0.01.