Abstract
Arsenic sulfide (As4S4) is the main component of realgar, which is widely used in traditional Chinese medicine. Previous studies have shown the beneficial effects of As4S4 in the treatment of hematological malignant diseases, however, its effects on solid tumors have yet to be fully elucidated. The current study aimed to explore the anti-cancer effect and the mechanism of As4S4 on solid tumors in vitro and in vivo. Cells from four human solid tumor cell lines, including the MKN45 gastric cancer cell line, the A375 malignant melanoma cell line, the 8898 pancreatic carcinoma cell line and the HepG2 hepatocellular carcinoma cell line, were treated with As4S4 in vitro, using the L02 embryonic liver cells as a control. The efficacy of As4S4 was assessed in vivo using mice implanted with Lewis lung carcinoma cells. The results of the current study demonstrated that As4S4 significantly inhibited the proliferation of solid tumor cells in a dose- and time-dependent manner, but produced a less pronounced effect on L02 cells. Additionally, As4S4 was observed to induce apoptosis (including morphological changes and an enhanced sub-G1 population), which was accompanied by the activation of caspase-3 and −9. Furthermore, treatment with As4S4 significantly inhibited the growth of implanted tumors in mice. These results suggest that As4S4 possesses potent in vitro and in vivo antitumor activity via the induction of cell apoptosis.
Keywords: arsenic sulfide, anti-cancer activity, solid tumor
Introduction
As a traditional Chinese medicine, arsenic has been widely used for over 2,000 years. It is effectively used in traditional remedies for the treatment of inflammation, ulcers, convulsions and schistosomiasis, and studies have demonstrated that arsenic produces positive effects in cancer therapy (1–3). One study demonstrated that arsenic trioxide (As2O3) was clinically effective in patients with acute promyelocytic leukemia (APL) (4). As2O3 was approved for the therapy of APL in the year 2000, and subsequently has been widely used therapeutically in liver, cervical and esophageal solid tumors (5–7). A number of studies have demonstrated that the induction of apoptosis and inhibition of proliferation are involved in the antitumor mechanism of As2O3 in hematopoietic malignancies and solid tumors (8–11).
Arsenic sulfide (As4S4), another arsenic compound, is the main active component of realgar, an orange-red crystalline mineral that has been extensively used in traditional Chinese medicine. Compared with As2O3, As4S4 is less toxic, but may elicit a similar anti-neoplastic action. The therapeutic potential of arsenic sulfide in malignancies, particularly hematopoietic tumors, has been the focus of a number of studies (12–15). Its antitumor effects are associated with the inhibition of proliferation, apoptosis and the suppression of BCR-ABL oncoprotein activity (16,17). However, the action of As4S4 as a treatment for solid tumor is unclear. Thus, the aim of the current study was to investigate the role of As4S4 in the treatment of solid tumors and its potential as an anticancer agent. In the present study, the anti-cancer effects of As4S4 were investigated in vitro and in vivo.
Materials and methods
Materials
A total of 6 g As4S4 was dissolved in 200 ml RPMI 1640 medium (Gibco Life Technologies, Carlsbad, CA, USA) for 12 h, then the concentration of arsenic was measured by atomic absorption spectrometry (IRIS 1000, Thermo, Waltham, MA, USA). The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cells and animals
The MKN45 gastric cancer, A375 malignant melanoma, 8898 pancreatic carcinoma, HepG2 hepatocellular carcinoma and L02 embryonic liver cell lines were purchased from the cell bank of the Type Culture Collection of The Chinese Academy of Sciences (Shanghai, China). All cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco Life Technologies), penicillin (100 U/ml) and streptomycin (100 U/ml; both Gibco Life Technologies). Cells were incubated at 37°C in a humidified atmosphere of 95% air and 5% CO2. Male C57BL/6 mice (n=32; age, six weeks), were obtained from the Animal Center of Fudan University (Shanghai, China; license no., 2007-0002 SCXK). Mice implanted with Lewis lung carcinoma (LLC) cells were purchased from the Institute of Materia Medica, Chinese Academy of Sciences (Shanghai, China; license no., SCXK 2004-0002). Mice were maintained in an animal facility under pathogen-free conditions (license no., SYXK 2003-0031). This study was approved by the ethics committee of Xin Hua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (Shanghai, China).
Cytotoxicity assay
The cytotoxicity assay was performed using MTT. Cells were seeded into and allowed to attach to 96-well culture plates (1×104 cells/well), for 4 h prior to treatment. As4S4 at concentrations of 0, 1.25, 2.5, 5 and 10 μg/ml was administered to the cells. After 24-h treatment, cell viability was evaluated by MTT assay. MTT solution (20 μl; Sigma-Aldrich) was added to each well and the plates were incubated for an additional 4 h at 37°C. The supernatant from each well was then carefully removed, and 100 μl dimethyl sulfoxide (Sigma-Aldrich) was added to each well and thoroughly mixed. The optical density (OD) was measured on a Model 550 microplate reader (Bio-Rad Laboratories, Hercules, CA, USA) at an absorbance wavelength of 492 nm and a reference wavelength of 630 nm. It was denoted that the percentage of cell viability = (average OD of experimental group/average OD of control group) × 100%. The experiment was repeated three times. The IC50 (concentration causing 50% inhibition of cell growth compared with the control) value of As4S4 for each of the tumor cell lines was also calculated after 24 h.
Determination of time-activity curve
The effect of As4S4 on cell viability was determined by measuring the MTT absorbance of living cells, which were seeded in and allowed to attach to 96-well plates. Following 0, 6, 12, 24, 36 and 48 h incubation of the tumor cells with As4S4 (at IC50) while L02 cells with 10 μg/ml As4S4, the cell viability was evaluated by MTT assay. The experiment was repeated three times.
Hematoxylin and eosin (HE) staining assay
Cells from exponentially growing cultures were seeded in 24-well culture plates and treated with As4S4 (at IC50) for 36 h, and L02 cells were treated with 10 μg/ml As4S4. Cells were washed with phosphate-buffered saline (PBS; Gibco Life Technologies), fixed in 4% paraformaldehyde (Sigma-Aldrich) for 15 min, stained with hematoxylin (Beyotime Institute of Biotechnology, Jiangsu, China) for 8 min, washed again with PBS, stained with eosin (Beyotime Institute of Biotechnology) for 5 min, and then examined and imaged with with the Nikon Eclipse 55i microscope (Nikon Corporation, Tokyo, Japan).
Flow cytometric analysis of cellular DNA content
Three cell lines (A375, MKN45 and L02) were seeded in 6-well culture plates (2×105 cells/well). Following 12-h incubation, A375 and MKN45 cells were treated with the respective IC50 of As4S4 for 36 h, and L02 cells were treated with 10 μg/ml As4S4. Floating and attached cells were collected in centrifuge tubes. Cells were washed with PBS, then resuspended and fixed in 70% ice-cold ethanol (Yangyuan, Changshu, China) for 4 h at 4°C. Subsequently, they were treated with RNase A (50 μg/ml; Sigma-Aldrich) for 30 min. Cells were stained with propidium iodide (50 μg/ml), then analyzed in a flow cytometer (BD Accuri C6, BD Biosciences, Franklin Lakes, NJ, USA). The percentages of cells in the G0/G1, S, G2/M and sub-G1 phases were analyzed using standard ModiFit LT 3.1and CellQuest Pro software (BD, Mac OS X.1; San Jose, CA, USA).
Lactate dehydrogenase (LDH) release assay
The A375 and MKN45 cells were treated with the respective IC50 doses of As4S4, and L02 cells were treated with 10 μg/ml As4S4. After 36 h, supernatants were harvested to measure the levels of LDH using the LDH kit (BHKT Clinical Reagent Co., Beijing, China)
Caspase activity assay
MKN45 cells were seeded in 10-cm dishes. Following a resting period of 12 h, cells were treated with various concentrations (0 μg/ml, 0.25 × IC50, 0.5 × IC50, 1 × IC50) of As4S4 for 36 h. Following treatment, the cells (floating and attached) were collected and washed three times with PBS and resuspended in 50 mM Tris-HCl (pH 7.4; Sigma-Aldrich), 1 mM EDTA (Sigma-Aldrich) and 10 mM ethyleneglycoltetraacetic acid (Sigma-Aldrich). Cell lysates were clarified by centrifugation at 18,000 × g for 3 min and clear lysates containing 50 μg protein were incubated with 100 μM enzyme-specific colorigenic substrates (Ac-DEVD-pNA; Beyotime Institute of Biotechnology) at 37°C for 1 h. The activity of caspase-3 and −9 was denoted as the cleavage of colorimetric substrate measured at an absorbance of 405 nm.
In vivo experiments with C57BL/6 mice
The mice implanted with LLC cells were sacrificed by cervical dislocation. Under sterile conditions, tumor tissues were dissected and the tumor cells were suspended in RPMI 1640 medium containing 10% FBS. The cell suspension was injected into the flanks of the experimental mice (106 cells in 200 μl PBS for each mouse). The tumor-bearing mice were divided into four groups: Negative control (NC) group, positive control group and high- and low- dose groups, each containing eight mice. The tumor-bearing mice were administered with an intraperitoneal injection of either 30 (low) or 60 (high) mg/kg dose of As4S4, daily for eight days. The NC group was treated with 0.9% normal saline (Rongbai, Shanghai, China) and the positive group was treated with 20 mg/kg cyclophosphamide (CTX; Yili, Shanghai, China), respectively. Subsequent to euthanization, the solid tumors were harvested and weighed, and blood was drawn to measure the level of interleukin-2 (IL-2). The solid tumor weights were statistically analyzed. The rate of inhibition (RI) was calculated according to the following formula: RI = [(mean tumor weight of the NC group - mean tumor weight of the drug group)/mean tumor weight of the NC group] × 100%.
Statistical analysis
Each experimental value was expressed as the mean ± standard deviation. Statistical analysis was performed using Origin software, version 7.0 (OriginLab, Northampton, MA, USA) to evaluate the differences between groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Cytotoxic effect of As4S4 on tumor cells
The cytotoxicity assay results for As4S4 on the five cell lines are presented in Fig. 1. The data indicated that the cell proliferation was inhibited by As4S4 in a dose-dependent (Fig. 1A) and time-dependent (Fig. 1B) manner (P<0.001), and each cell line presented a different sensitivity to the inhibitory effect of As4S4. The IC50 values of the tumor cell lines following 24-h treatment are presented in Table I. As4S4 generated a weaker effect on L02 cells compared with the four tumor cell lines.
Figure 1.
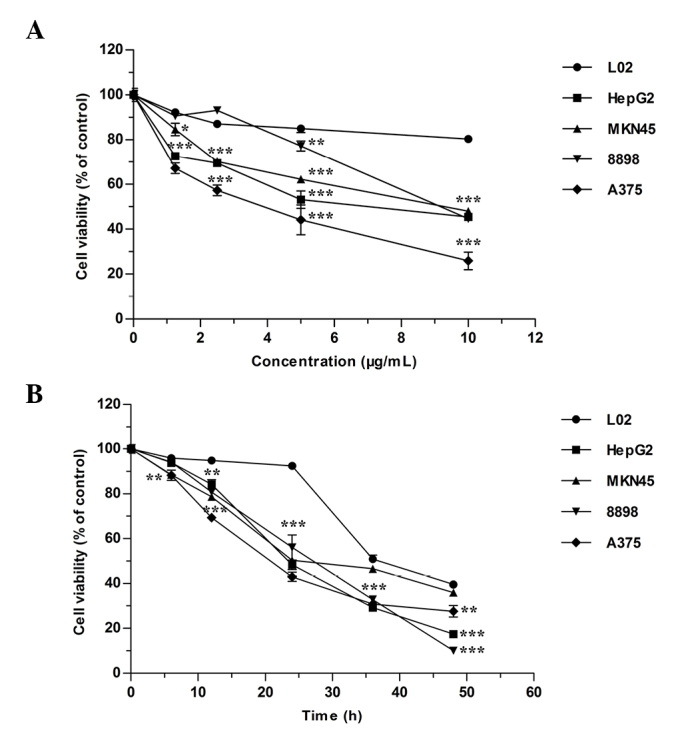
Cytotoxic effect of As4S4 on 8898, A375, HepG2 and MKN45 cancer cells and L02 healthy liver cells. (A) Viability of the cells was measured by MTT assay following exposure to As4S4 at different concentrations (1.25–10 μg/ml) for 24 h. (B) Measurement of cell viability following treatment with the respective IC50s of As4S4 for each tumor cell line for different time periods (0–48 h) (L02 cells were treated with 10 μg/ml As4S4). Data are expressed as the mean ± standard deviation. The cell proliferation was inhibited by As4S4 in an (A) dose-dependent and (B) time-dependent manner (P<0.001) and each cell line presented a different sensitivity to the inhibitory effect of As4S4 (*P<0.05, **P<0.01 and ***P<0.001, vs. L02 cells). As4S4, arsenic sulfide.
Table I.
IC50 value of arsenic sulfide for each tumor cell line following 24-h treatment.
| Cell line | IC50 (μg/ml) × ± standard deviation |
|---|---|
| HepG2 | 6.89±1.078 |
| MKN45 | 9.37±0.948 |
| 8898 | 9.06±0.984 |
| A375 | 3.78±0.827 |
Effect of As4S4 on cell morphology
The HE staining assay identified that the tumor cells (8898, A375, HepG2 and MKN45) treated with As4S4 exhibited cell shrinkage, nuclear condensation and fragmentation, which are typical characteristics of apoptosis. However, the treated L02 cells did not exhibit significant morphological changes (Fig. 2).
Figure 2.
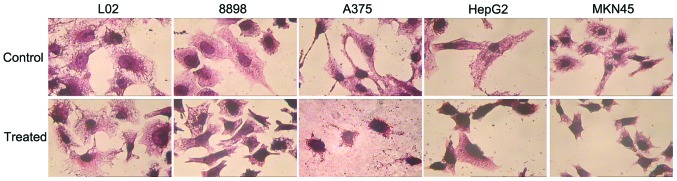
Hematoxylin and eosin staining of 8898, A375, HepG2 and MKN45 cells cultured in the presence or absence of the respective IC50s of As4S4 for 36 h and L02 cells treated with 10 μg/ml As4S4. Condensed and fragmented nuclei were observed in the As4S4-treated tumor cells, but not in the L02 cells. Magnification, ×400. As4S4, arsenic sulfide.
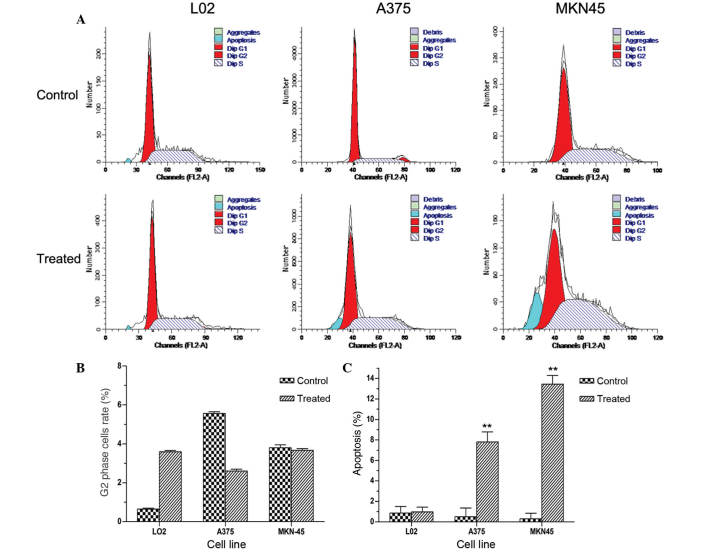
Effect of As4S4 on G2/M phase arrest and the apoptotic sub-G1 population
To determine whether the reduction in cell viability observed involved alterations to the cell cycle, the effect of As4S4 on the cell cycle distribution in the A375, MKN45 and L02 cell lines was investigated using fluorescence-activated cell sorting analysis (Fig. 3A). The apoptotic index was calculated by measuring the number of cells in the sub-G1 population following treatment with As4S4. Subsequent to exposure of A375, MKN45 cells to the respective IC50s of As4S4 and of L02 cells to 10 μg/ml As4S4 for 36 h, no marked G2/M phase arrest was observed, as demonstrated in Fig. 3B. These results suggest that As4S4 produced no significant effect on G2/M phase arrest. A significant increase in the sub-G1 fraction was identified in tumor cells treated with As4S4, whilst no significant difference was observed in the L02 cells compared with the untreated control cells (Fig. 3C).
Figure 3.
Effect of As4S4 on G2/M phase and apoptotic cells. (A) L02, A375 and MKN45 cells were treated with or without As4S4 and analyzed by flow cytometry. (B) Data indicated no clear G2/M phase arrest in the tumor or L02 cells treated with As4S4. (C) A significant increase in apoptotic sub G1 population was observed in As4S4-treated A375 and MKN45 cells, but not in the L02 cells. Data are expressed as the mean ± standard deviation; *P<0.05, **P<0.01 and ***P<0.001 vs. control. As4S4, arsenic sulfide.
Effect of As4S4 on the level of LDH and the activation of caspase
The LDH release assay measured the leakage of LDH into the extracellular medium following cellular lysis. The release of intracellular LDH was detected following exposure to As4S4 (Fig. 4A). Meanwhile, caspase-3 and −9 were activated in MKN45 cells treated with As4S4 for 36 h (Fig. 4B). These results indicate the involvement of caspase-3 and −9 in As4S4-mediated cell apoptosis.
Figure 4.
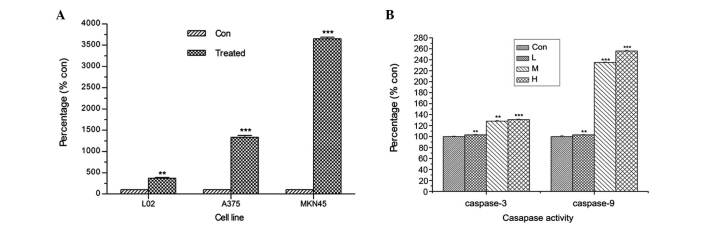
Effect of As4S4 on the release of LDH and the activation of caspase-3 and −9. (A) Subsequent to exposure of A375 and MKN45 cells to the respective IC50s of As4S4 for 36 h, and exposure of L02 cells to 10 μg/ml As4S4, the leakage of LDH from cells was analyzed. (B) Caspase-3 and −9 activation was analyzed in MKN45 cells treated with different concentrations of As4S4 for 36 h: Control, 0 μg/ml; L, 0.25 × IC50; M, 0.5 × IC50 and H, 1 × IC50. Data are expressed as the mean ± standard deviation; *P<0.05, **P<0.01 and ***P<0.001 vs. control. As4S4, arsenic sulfide; LDH, lactate dehydrogenase; L, low; M, medium; H, high.
As4S4 inhibits the growth of solid tumors and elevates the levels of IL-2 in blood
Mouse lung cancer LLC cells were implanted in C57BL/6 mice. Following treatment with As4S4 for eight days, the suppression of tumor growth was observed (Fig. 5A). The inhibition ratios of the low-dose group (As4S4, 30 mg/kg) and high-dose group (As4S4, 60 mg/kg) were 26.45 and 47.93%, respectively (Table II). The high dosage exhibited a greater anticancer effect than the low dosage, and produced a significantly reduced tumor weight compared with that of the NC group. In addition, As4S4 treatment did not significantly alter the body weight of the mice (data not shown). Subsequent to treatment with As4S4, the concentrations of IL-2 in the treatment groups were higher compared with the NC group (Fig. 5B). These results demonstrate that As4S4 is able to suppress tumor growth.
Figure 5.
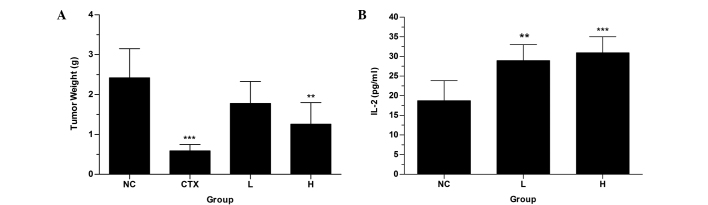
As4S4 inhibited tumor growth and elevated the levels of IL-2 in the tumor-bearing C57BL/6 mice. (A) Tumor weight reduced significantly following treatment with 60 mg/kg (H) of As4S4, but the reduction observed was not significant with 30 mg/kg (L). (B) Levels of IL-2 were significantly elevated in the treatment groups compared with the NC group. Data are expressed as the mean ± standard deviation; *P<0.05, **P<0.01 and ***P<0.001 vs. NC. As4S4, arsenic sulfide; IL-2, interleukin 2; L, low; H, high; NC, negative control; CTX, cyclophosphamide.
Table II.
Antitumor effect of As4S4 in vivo.
| Group | Injection dosage (mg/kg) × ± standard deviation | Tumor weight (g) | Tumor inhibition (%) |
|---|---|---|---|
| Negative control | 0 | 2.42±0.73 | 0 |
| Positive control (cyclophosphamide) | 20 | 0.59±0.16b | 75.62 |
| High As4S4 | 60 | 1.26±0.54a | 47.93 |
| Low As4S4 | 30 | 1.78±0.55 | 26.45 |
C57BL/6 mice were inoculated with Lewis lung carcinoma cells (106 cells/mouse), injected intraperitoneally with As4S4 for eight days and subsequently sacrificed. Data are expressed as the mean ± standard deviation.
P<0.01;
P<0.001 vs. negative control.
As4S4, arsenic sulfide.
Discussion
As4S4 has attracted worldwide interest in recent years due to the successful clinical application of arsenic compounds in the treatment of APL (12,18). However, its efficacy in the treatment of solid tumors remains to be thoroughly elucidated. Hence, in the present study, the antitumor effect of As4S4 on solid tumors and its possible mechanism of action were investigated.
Apoptosis, the most common form of tumor cell death, is a biological process of programmed cell death (PCD). The typical morphological and molecular changes that occur during the course of apoptosis include cell shrinkage, nuclear fragmentation, chromatin condensation, DNA fragmentation and changes in apoptotic protein expression (19–21). In the current study, using MTT assay, As4S4 was observed to be able to inhibit the proliferation of tumor cells in a dose- and time-dependent manner, but produced a less marked effect on healthy L02 cells, which were used as a control to assess the hepatotoxicity of As4S4. Flow cytometry indicated that the inhibition of A375 and MKN45 tumor cell growth by As4S4 may be due to cell apoptosis. However, no significant effect of As4S4 on G2/M phase arrest was observed. The HE staining assay demonstrated that four tumor cell lines treated with the IC50 of As4S4 for 36 h exhibited cell shrinkage, nuclear condensation and fragmentation (typical characteristics of apoptosis), while L02 cells treated with As4S4 demonstrated no differences prior to and post administration.
LDH is clinically significant as a marker of injury and disease, since it is released during cell or tissue damage. The LDH release assay measures the leakage of the soluble cytoplasmic LDH enzyme into the extracellular medium via cellular lysis. The current study demonstrated that As4S4 increased the release of LDH, indicating the induction of apoptosis or necrosis.
Caspases are cysteine proteases activated by a cascade, involving the cleavage of their precursors. Caspase-3 is an executioner caspase that disassembles cells through the cleavage of proteins such as PARP, in order to inactivate them. Caspase-3 is commonly activated by either the caspase-9-mediated mitochondrial pathway, or by the caspase-8-mediated death receptor pathway (22–24). In the current study, As4S4 treatment was demonstrated to lead to caspase-3 and −9 activation, which suggests the involvement of the caspase pathways in As4S4-induced apoptosis.
The antitumor activity of As4S4 was further examined in a mouse model. Similar to the in vitro results, As4S4 was capable of inhibiting tumor growth in vivo. In the high dosage group, the tumors grew slower compared to the NC group. However, As4S4 did not produce a significant inhibitory effect on tumor growth in the low As4S4 dosage group compared with the NC group.
Studies have demonstrated that IL-2 serves an important function in the regulation of antigen-specific T-cell responses (25,26). The cytokines expressed by a T-cell in response to an antigen indicate the specific pathway, and IL-2 is associated with the T helper 1 (Th1) pathway. IL-2 has been demonstrated to serve an important role in specific immunological responses to tumor cell growth (27,28). Thus, in the present study, IL-2 production was investigated to evaluate the hypothesis that As4S4 increases T-cell activation by modulating IL-2. The results demonstrated that the serum concentrations IL-2 were enhanced in As4S4-treated C57BL/6 mice bearing LLC, compared with the NC mice, suggesting that it may be a potent inducer of Th1-type cytokines.
In the present study, As4S4 was observed to induce apoptosis in cancer cells. The detailed mechanism of apoptosis induction in solid tumors by As4S4 requires further investigation. Previous studies have reported that As4S4 treatment induced differentiation of hematological tumor cells (29,30), suggesting that the antitumor action of As4S4 in solid tumors may involve cellular differentiation.
In the current study, the results suggested that As4S4 may be able to inhibit cell growth and increase the release of LDH. Furthermore, the apoptosis was suggested to involve caspase-3 and −9 activation. Additionally, As4S4 was demonstrated to have the effect of suppressing tumor growth in vivo. These results suggest that As4S4 may be a potential therapeutic candidate in the treatment of solid tumors.
Acknowledgements
The current study was supported by the National Natural Science Foundation of China (grant nos. 81274142 and 30300139); the Science and Technology Commission of Shanghai Municipality (grant no. 11ZR1423400); the Key Project of Shanghai Municipal Education Commission (grant no. 07zz43); and Shanghai Jiao Tong University School of Medicine Foundation of Science and Technology (05XJ21030).
References
- 1.Nakagawa Y, Akao Y, Morikawa H, et al. Arsenic trioxide-induced apoptosis through oxidative stress in cells of colon cancer cell lines. Life Sci. 2002;70:2253–2269. doi: 10.1016/S0024-3205(01)01545-4. [DOI] [PubMed] [Google Scholar]
- 2.Ramanathan K, Anusuyadevi M, Shila S, Panneerselvam C. Ascorbic acid and alpha-tocopherol as potent modulators of apoptosis on arsenic induced toxicity in rats. Toxicol Lett. 2005;156:297–306. doi: 10.1016/j.toxlet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Waxman S, Anderson KC. History of the development of arsenic derivatives in cancer therapy. Oncologist. 2001;6(Suppl 2):3–10. doi: 10.1634/theoncologist.6-suppl_2-3. [DOI] [PubMed] [Google Scholar]
- 4.Niu C, Yan H, Yu T, et al. Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood. 1999;94:3315–3324. [PubMed] [Google Scholar]
- 5.Murgo AJ. Clinical trials of arsenic trioxide in hematologic and solid tumors: overview of the National Cancer Institute Cooperative Research and Development Studies. Oncologist. 2001;6(Suppl 2):22–28. doi: 10.1634/theoncologist.6-suppl_2-22. [DOI] [PubMed] [Google Scholar]
- 6.Zheng J, Deng YP, Lin C, Fu M, Xiao PG, Wu M. Arsenic trioxide induces apoptosis of HPV16 DNA-immortalized human cervical epithelial cells and selectively inhibits viral gene expression. Int J Cancer. 1999;82:286–292. doi: 10.1002/(SICI)1097-0215(19990719)82:2<286::AID-IJC21>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 7.Shen ZY, Zhang Y, Chen JY, et al. Intratumoral injection of arsenic to enhance antitumor efficacy in human esophageal carcinoma cell xenografts. Oncol Rep. 2004;11:155–159. [PubMed] [Google Scholar]
- 8.Emadi A, Gore SD. Arsenic trioxide - An old drug rediscovered. Blood Rev. 2010;24:191–199. doi: 10.1016/j.blre.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rojewski MT, Baldus C, Knauf W, Thiel E, Schrezenmeier H. Dual effects of arsenic trioxide (As2O3) on non-acute promyelocytic leukaemia myeloid cell lines: induction of apoptosis and inhibition of proliferation. Br J Haematol. 2002;116:555–563. doi: 10.1046/j.0007-1048.2001.03298.x. [DOI] [PubMed] [Google Scholar]
- 10.Park WH, Seol JG, Kim ES, et al. Arsenic trioxide-mediated growth inhibition in MC/CAR myeloma cells via cell cycle arrest in association with induction of cyclin-dependent kinase inhibitor, p21, and apoptosis. Cancer Res. 2000;60:3065–3071. [PubMed] [Google Scholar]
- 11.Zhong F, Zhang S, Shao C, Yang J, Wu X. Arsenic trioxide inhibits cholangiocarcinoma cell growth and induces apoptosis. Pathol Oncol Res. 2010;16:413–420. doi: 10.1007/s12253-009-9234-1. [DOI] [PubMed] [Google Scholar]
- 12.Lu DP, Qiu JY, Jiang B, et al. Tetra-arsenic tetra-sulfide for the treatment of acute promyelocytic leukemia: a pilot report. Blood. 2002;99:3136–3143. doi: 10.1182/blood.V99.9.3136. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Zhou GB, Liu P, et al. Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proc Natl Acad Sci USA. 2008;105:4826–4831. doi: 10.1073/pnas.0712365105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang QY, Mao JH, Liu P, et al. A systems biology understanding of the synergistic effects of arsenic sulfide and Imatinib in BCR/ABL-associated leukemia. Proc Natl Acad Sci USA. 2009;106:3378–3383. doi: 10.1073/pnas.0813142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen S, Fang Y, Ma L, Liu S, Li X. Realgar-induced apoptosis and differentiation in all-trans retinoic acid (ATRA)-sensitive NB4 and ATRA-resistant MR2 cells. Int J Oncol. 2012;40:1089–1096. doi: 10.3892/ijo.2011.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin T, Wu YL, Sun HP, et al. Combined effects of As4S4 and imatinib on chronic myeloid leukemia cells and BCR-ABL oncoprotein. Blood. 2004;104:4219–4225. doi: 10.1182/blood-2004-04-1433. [DOI] [PubMed] [Google Scholar]
- 17.Wu J, Shao Y, Liu J, Chen G, Ho PC. The medicinal use of realgar (As4S4) and its recent development as an anticancer agent. J Ethnopharmacol. 2011;135:595–602. doi: 10.1016/j.jep.2011.03.071. [DOI] [PubMed] [Google Scholar]
- 18.Lu DP, Wang Q. Current study of APL treatment in China. Int J Hematol. 2002;76(Suppl 1):316–318. doi: 10.1007/BF03165273. [DOI] [PubMed] [Google Scholar]
- 19.Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- 20.McConkey DJ, Orrenius S. Signal transduction pathways in apoptosis. Stem Cells. 1996;14:619–631. doi: 10.1002/stem.140619. [DOI] [PubMed] [Google Scholar]
- 21.Hunot S, Flavell RA. Apoptosis. Death of a monopoly? Science. 2001;292:865–866. doi: 10.1126/science.1060885. [DOI] [PubMed] [Google Scholar]
- 22.Hengartner M. Apoptosis. Death by crowd control. Science. 1998;281:1298–1299. doi: 10.1126/science.281.5381.1298. [DOI] [PubMed] [Google Scholar]
- 23.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 24.McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5:a008656. doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988;240:1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 26.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 27.McAdam AJ, Pulaski BA, Harkins SS, Hutter EK, Lord EM, Frelinger JG. Synergistic effects of co-expression of the TH1 cytokines IL-2 and IFN-gamma on generation of murine tumor-reactive cytotoxic cells. Int J Cancer. 1995;61:628–634. doi: 10.1002/ijc.2910610508. [DOI] [PubMed] [Google Scholar]
- 28.Sakthivel KM, Guruvayoorappan C. Acacia ferruginea inhibits tumor progression by regulating inflammatory mediators-(TNF-α, iNOS, COX-2, IL-1β, IL-6, IFN-γ, IL-2, GM-CSF) and pro-angiogenic growth factor-VEGF. Asian Pac J Cancer Prev. 2013;14:3909–3919. doi: 10.7314/APJCP.2013.14.6.3909. [DOI] [PubMed] [Google Scholar]
- 29.Wang LW, Shi YL, Wang N, Gou BD, Zhang TL, Wang K. Association of oxidative stress with realgar-induced differentiation in human leukemia HL-60 cells. Chemotherapy. 2009;55:460–467. doi: 10.1159/000265528. [DOI] [PubMed] [Google Scholar]
- 30.Wang N, Wang LW, Gou BD, Zhang TL, Wang K. Realgar-induced differentiation is associated with MAPK pathways in HL-60 cells. Cell Biol Int. 2008;32:1497–1505. doi: 10.1016/j.cellbi.2008.08.017. [DOI] [PubMed] [Google Scholar]