Figure 4.
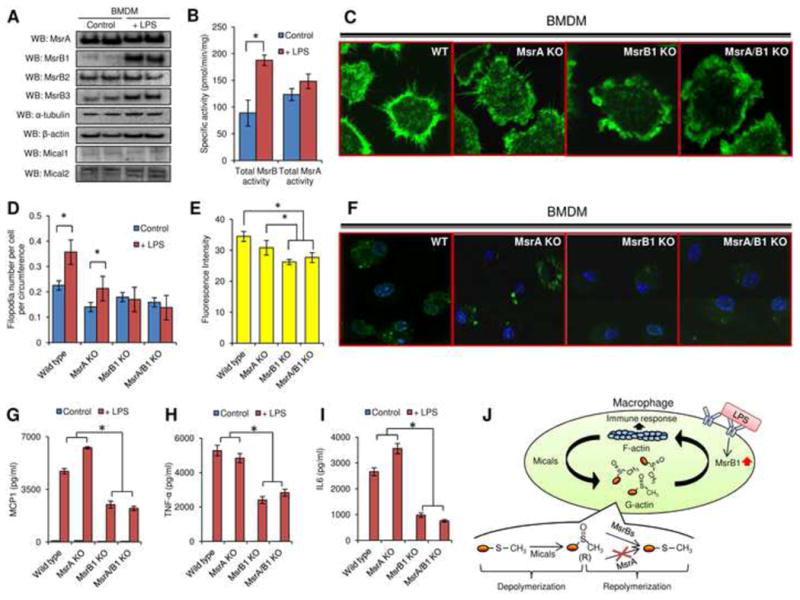
Regulation of actin polymerization-dependent processes by MsrB1 in bone marrow derived macrophages. (A) MsrA, MsrB1, MsrB2, MsrB3, α-tubulin, β-actin, Mical1, and Mical2 expression was analyzed by Western blots in control and LPS-stimulated cells. (B) Total MsrB and MsrA activities were measured by HPLC in bone marrow derived macrophages before and after LPS stimulation (n=3). (C) Filopodia formation and (F) Macropinocytosis were evaluated in LPS-primed macrophages generated from the bone marrow of wild type, MsrA KO, MsrB1 KO, and MsrA/MsrB1 KO mice. For macropinocytosis assays, M-CSF was used to stimulate the internalization of lucifer yellow fluorescence and only LPS-primed macrophages were included since unprimed macrophages did not exhibit detectable dye uptake. Quantitation of (D) filopodia number per cell per circumference (n=7) and (E) internalized lucifer yellow before in LPS primed macrophages is shown for wild type, MsrA KO, MsrB1 KO, MsrA/MsrB1 KO mice (n=4). (*: p < 0.05). Calculation of filopodia number is described in detail in Fig. S4C. (G) MCP1, (H) TNF-α, and (I) IL6 secretion before and after LPS stimulation from bone marrow derived macrophages generated from wild type, MsrA KO, MsrB1 KO, MsrA/MsrB1 KO mice was analyzed (n=4) (*: p < 0.05). (J) A model of redox regulation of actin function via reversible stereoselective site-specific Met oxidation and reduction and its importance for the macrophage immune response. All used error bars represent SD. See also Figures S3 and S4.
