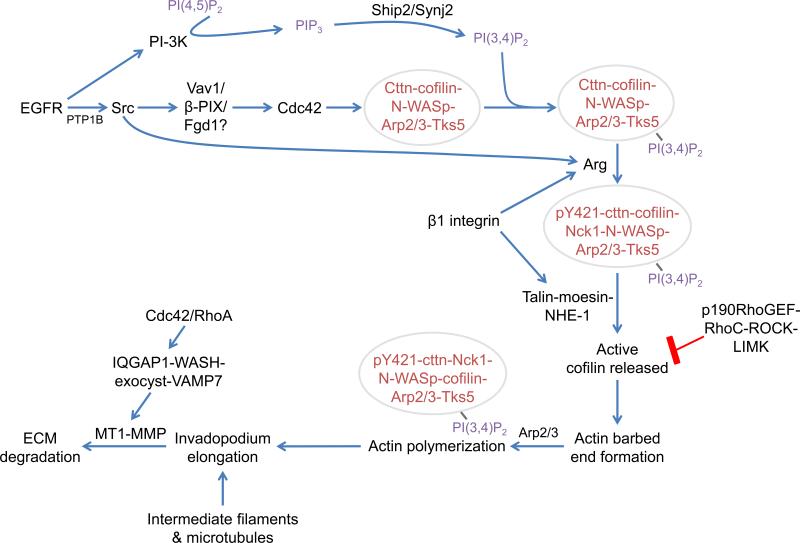
Figure 2. Integrative signaling diagram of invadopodial assembly and maturation.
Invadopodia initially form as precursors in response to EGF or other stimuli (e.g. TGF-β or PDGF). Src is activated either directly by EGFR or by PTP1B (Cortesio et al., 2008). These stimuli induce Cdc42 activation, leading to assembly of the precursor core structure (red text within circle; Razidlo et al., 2014; Yamaguchi et al., 2005). The invadopodium precursor is then anchored by binding to PI(3,4)P2 and further stabilized by β1 integrin-mediated adhesion to the ECM (Beaty et al., 2013; Sharma et al., 2013). Invadopodium maturation begins as β1 integrin activates Arg, which phosphorylates cortactin on Y421 to recruit Nck1 (Beaty et al., 2013; Oser et al., 2010). Talin localizes to the structure and recruits a complex of moesin and NHE-1 through a direct binding interaction with moesin (Beaty et al., 2014). The intracellular pH increases in response to NHE-1 activity, which disrupts the inhibitory interaction between cortactin and cofilin (Busco et al., 2010; Magalhaes et al., 2011). Cofilin severs F-actin to form barbed ends that are used to elongate filaments, on which Nck1 induces N-WASp-Arp2/3-dependent dendritic nucleation (DesMarais et al., 2004). Actin polymerization is required for MMP-dependent matrix degradation at invadopodia, possibly through MMP recruitment (Oser et al., 2009; Sakurai-Yageta et al., 2008; Yamaguchi et al., 2005). MT1-MMP is delivered by the IQGAP1-WASH-exocyst complex and fuses to the membrane via the v-SNARE VAMP7, resulting in matrix degradation (Monteiro et al., 2013; Sakurai-Yageta et al., 2008; Steffen et al., 2008). Cortactin, cttn; synaptojanin2, synj2.