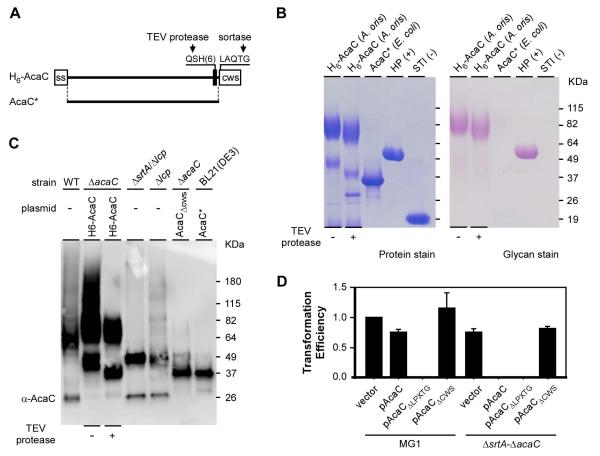
Figure 5.
AcaC is a cell wall anchored glycosylated protein. (A) Shown is a diagram of a recombinant AcaC protein with its signal sequence (ss), TEV cleavage site followed by a six-histidine tag, and cell wall sorting signal (CWS) with the LAQTG motif. A truncated version of AcaC lacking the signal sequence (ss) and CWS is denoted as AcaC*. (B) Purified AcaC proteins from E. coli and A. oris were separated by SDS-PAGE and stained with Coomassie (protein stain) or Periodic acid–Schiff (glycan stain). Nonglycosylated soybean trypsin inhibitor (STI) and glycosylated horseradish peroxidase (HP) were used as controls. (C) The His-tagged AcaC proteins purified from A. oris in B were treated with TEV protease and subjected to immunoblotting with α-AcaC; their mobility was compared with AcaC species isolated from cell wall fractions of the MG1 (WT) and Δlcp mutant strains, culture medium fractions of mutants ΔsrtA/Δlcp and ΔacaC/pAcaCΔcws, and truncated AcaC purified from E. coli BL21(DE3). (D) Transformability of the empty vector and plasmids expressing full-length AcaC (pAcaC), AcaC lacking the LPXTG motif (ΔLPXTG), or AcaC lacking the CWS in strains MG1 and its isogenic mutant ΔsrtA-ΔacaC was determined by cell growth as described in Fig. 3B. The transformation efficiency values, averages of three independent experiments, are presented relative to that of the empty vector in MG1, which was arbitrarily assigned as 1.