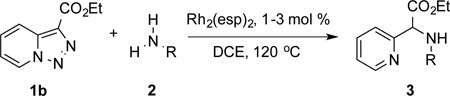
Table 2.
Substrate Scope for the Rh(II)-Catalyzed Reaction of Pyridotriazoles with Anilines and Aliphatic Amines.[a,b]
 | |||||
|---|---|---|---|---|---|
| Entry | Product | Yield, % |
Entry | Product | Yield, % |
| 1 | 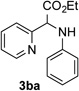 |
88 | 7 | 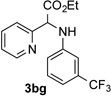 |
71 |
| 2 | 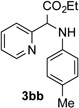 |
63 | 8 | 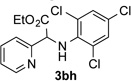 |
90 |
| 3 | 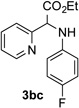 |
80 | 9 | 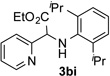 |
47 |
| 4 | 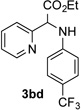 |
86 | 10 | 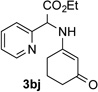 |
91[c] |
| 5 | 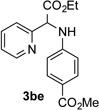 |
72 | 11 | 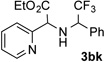 |
87 |
| 6 | 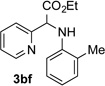 |
76 | 12 | 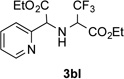 |
82 |
Conditions: triazole 1 (0.20 mmol), N–H compounds 2 (1.5 equiv.), and Rh2(esp)2 (3.0 mol %) were heated in 2 ml of dry DCE at 120 °C until completion.
Isolated yields.
1.0 mol % of Rh2(esp)2.
