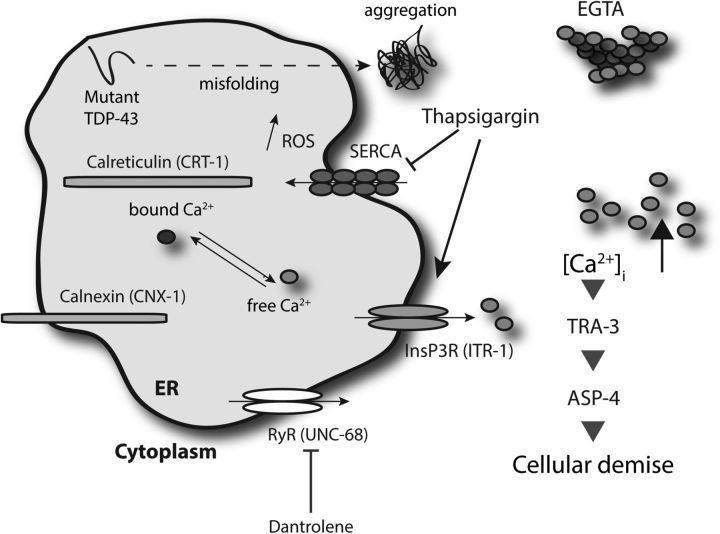
Figure 8.
Working model for TDP-43 and Ca2+-dependent necrosis-like neuronal toxicity. The chronic stress induced by protein misfolding and aggregation of mutant TDP-43 proteins may lead to inappropriate release of Ca2+ from ER stores into the cytoplasm. The resultant [Ca2+]i increase is essential for downstream events, including activation of the Ca2+-regulated TRA-3 calpain protease, which in turn mediates leakage of killer aspartyl proteases (ASP-4), leading to neuronal dysfunction and cell death. Mutations affecting ER Ca2+ storage (calreticulin and calnexin) or ER calcium release (InsP3R and RYR calcium release channels) disrupt release and are therefore neuroprotective. Pharmacological reduction of [Ca2+]i by EGTA or dantrolene is also neuroprotective, while a forced increase of [Ca2+]i by thapsigargin enhances neuronal toxicity. Disabling the activity of calpain or aspartyl proteases also protects against TDP-43-associated neuronal dysfunction and degeneration.