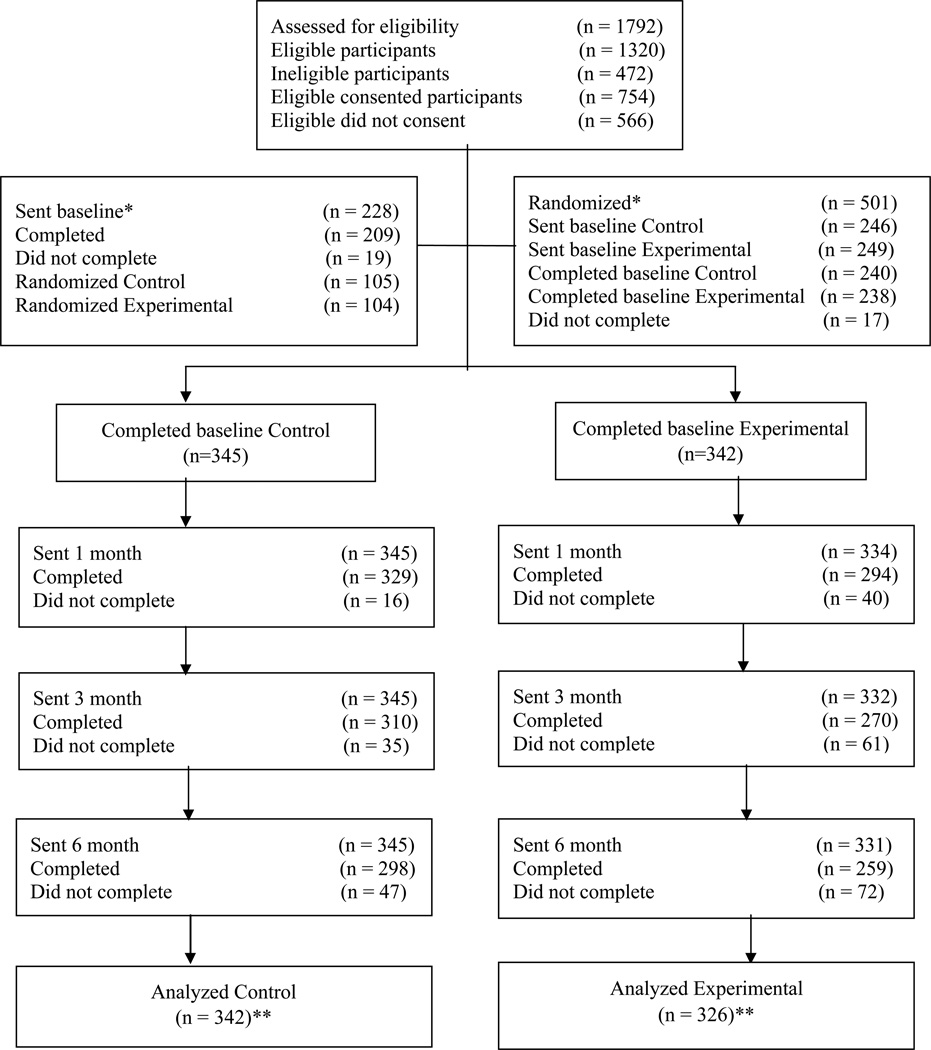
Figure 1.
Consolidated Standards of Reporting Trials flow diagram
* Participants in the back pain study were given baseline and then randomized. Participants in the arthritis and neuropathic pain studies were randomized and then given the baseline assessment. In the neuropathic pain study 25 eligible consented participants did not complete the background assessment and therefore were not randomized.
** For analysis, ten participants (1 control, 9 experimental) in the back pain study were removed from the study for various reasons (illness, illiterate, in multiple studies, no data, used for usability and used multiple names). Further, due to technical reasons data from 9 participants in the back pain study were missing, hence excluded from analysis (2 control, 7 experimental).