Abstract
To investigate the association between cannabis smoking and lung cancer risk, data on 2,159 lung cancer cases and 2,985 controls were pooled from 6 case-control studies in the US, Canada, UK, and New Zealand within the International Lung Cancer Consortium. Study-specific associations between cannabis smoking and lung cancer were estimated using unconditional logistic regression adjusting for sociodemographic factors, tobacco smoking status and pack-years; odds-ratio estimates were pooled using random effects models. Subgroup analyses were done for sex, histology and tobacco smoking status. The shapes of dose-response associations were examined using restricted cubic spline regression. The overall pooled OR for habitual versus nonhabitual or never users was 0.96 (95% CI: 0.66–1.38). Compared to nonhabitual or never users, the summary OR was 0.88 (95%CI: 0.63–1.24) for individuals who smoked 1 or more joint-equivalents of cannabis per day and 0.94 (95%CI: 0.67–1.32) for those consumed at least 10 joint-years. For adenocarcinoma cases the ORs were 1.73 (95%CI: 0.75–4.00) and 1.74 (95%CI: 0.85–3.55), respectively. However, no association was found for the squamous cell carcinoma based on small numbers. Weak associations between cannabis smoking and lung cancer were observed in never tobacco smokers. Spline modeling indicated a weak positive monotonic association between cumulative cannabis use and lung cancer, but precision was low at high exposure levels. Results from our pooled analyses provide little evidence for an increased risk of lung cancer among habitual or long-term cannabis smokers, although the possibility of potential adverse effect for heavy consumption cannot be excluded.
Keywords: lung cancer, Cannabis smoking, never smokers, marijuana smoking
Background
Cannabis is the world's most widely used illicit substance with between 119 million and 224 million users worldwide. In 2010, the proportion reporting use of cannabis in the past year was 2.6– 5% for persons aged 15–64, with highest frequency observed in Oceania (9.1–14.6%) and North America (10.8%).1 In the US, frequency of cannabis use has continued to rise2 especially among teenagers, a trend that has been attributed to falling perceived risk.1 The three main forms of cannabis products are the flower or herb (marijuana), resin (hashish), and oil (hashish oil), and their relative levels of consumption vary globally by region. Resin dominates the markets in the Near- and Middle-East as well as Southwest Asia; resin and herb markets are comparable in size in Northern Africa and Europe; whereas cannabis herb dominates the rest of the world including North America.1
Lung cancer remains the leading cause of cancer death worldwide, and use of tobacco is recognized as the main risk factor.3–5 Cannabis is mainly consumed by smoking, and cannabis smoke shares carcinogens with tobacco smoke including polycyclic aromatic hydrocarbons such as benzo[α]pyrene and phenols.5–7 Different smoking techniques result in 3-fold higher levels of tar and 5-fold higher levels of carbon monoxide being retained in the lungs during cannabis smoking as compared to tobacco smoking.8 Therefore, cannabis has been hypothesized to be a risk factor for tobacco-related cancers including that of the lung. Previous studies have demonstrated precancerous histological9,10 and molecular abnormalities11 in the respiratory tracts of cannabis smokers. In addition, in vitro12 and in vivo animal studies have demonstrated the carcinogenic effects of cannabis or its constituents.13,14 However, epidemiological studies investigating the association between cannabis smoking and lung cancer have been limited, sample sizes generally small, and results conflicting.15–22
Established in 2004, the International Lung Cancer Consortium (ILCCO) brings together an international group of lung cancer researchers with the aim of sharing comparable data from ongoing and recently completed lung cancer case-control and cohort studies from different geographical areas and ethnicities. One of the key goals of the ILCCO is to explore potential lung cancer risk factors that are difficult to evaluate in individual studies. To address the limitations of prior studies and to further explore the link between cannabis smoking and lung cancer development, particularly nonlinear dose-response relations and associations among never tobacco smokers and other subgroups, we conducted a pooled analysis based on individual-level data from participating ILCCO studies.
Methods
Data collection
Details of the International Lung Cancer Consortium and the requirements for inclusion of studies have been previously published23 and are available on the Consortium portal (http://ilcco.iarc.fr). Six ILCCO studies have collected information on cannabis smoking and contributed primary data in this pooled analysis investigating the association between cannabis smoking and lung cancer risk. Two studies had previously reported effect estimates for cannabis smoking,15,16 whereas the remaining studies represented unpublished data for the association of interest. All studies considered primary, incident and histologically confirmed lung cancer cases. Written informed consent was obtained from all study subjects, and individual study protocols were approved by site-specific institutional ethic review boards. Deidentified data received from individual studies were checked for missing values, inadmissible values, aberrant distributions and inconsistencies. Subjects with unknown age, sex, race or habitual versus nonhabitual cannabis smoking status were excluded. An additional 6 subjects, whose joints smoked per day exceeded 40 or lifetime duration of cannabis smoking exceeded 70 years, were deemed outliers or potential data errors based on the overall distribution. The final pooled analysis sample consisted of 2,159 cases and 2,985 controls.
Statistical methods
Data on individual-level cannabis smoking consumption were based on self-reported responses to questions on study-specific questionnaires. We defined lifetime habitual use of cannabis as having a cumulative consumption of at least 1 joint-year (i.e., equivalent to smoking 1 joint/day for 1 year). Joint-equivalent was defined as the average cannabis plant matter contained in a typical joint or 0.75 g/joint when the unit of reporting was weight or the mode of consumption was other than joint.24 We also harmonized variables pertaining to total duration (years) and intensity of cannabis smoking (average joint-equivalents smoked per day during periods of cannabis use); and cumulative cannabis smoking in joint-years, calculated by multiplying the first two variables for each subject.
Variables for demographic characteristics and well-established lung cancer risk factors were harmonized across studies. Self-reported tobacco smoking status at interview was defined as never smokers (<100 cigarettes over lifetime or according to study-specific cut-offs), former smokers (stopped smoking at least 2 years prior to interview), and current smokers (smoked within the past 2 years). The latter two categories make up the ever group in never versus ever definition for tobacco-smoking status. For current and former tobacco smokers, cumulative tobacco smoking expressed in pack-years was calculated as the product of smoking intensity (pack-equivalent per day) and the sum of smoking periods over the person's lifetime. When education level (<3%) or tobacco pack-years were missing (<5%), values were imputed using the median of the study-, age-, and sex-specific control population for education, and the median of the study-, age-, sex-, and smoking status-specific control population for pack-years.
The overall association between cannabis smoking (habitual vs. nonhabitual or never users, joint-equivalents per day, duration, total joint-years, and age of cannabis smoking onset) and the risk of all lung cancer, adenocarcinoma, and squamous cell carcinoma (as sample size permitted) was assessed by odds ratios (OR) and 95% confidence intervals (CI) obtained from unconditional logistic regression in each study, adjusting for age, sex, race, highest education, status of tobacco smoking (never vs. ever) and pack-years of tobacco smoking (continuous). Because of collinearity and small sample sizes, models for continuous exposure variables for the Moffitt Cancer Study and Memorial Sloan-Kettering Cancer Center Study were restricted to the adjustment of the essential covariates: age, sex and tobacco smoking pack-years. Tobacco smoking status (never vs. ever) was additionally adjusted where possible. Study-specific effect estimates were pooled across studies using random effect models to account for heterogeneity between study populations. Interstudy heterogeneity was evaluated based on the Q-statistic and I2 statistic.25 When the p-value for heterogeneity across studies was less than 0.05, influence analysis was performed to evaluate the source of heterogeneity from single studies using Galbraith plots,26 and excluding the study contributing most to the Q-statistic. Stratified analyses were done to examine the associations between cannabis smoking and all lung cancer for males and females separately. As an alternative method to control for confounding by tobacco smoking, as sample sizes allowed, we also conducted analyses restricted to those who never smoked tobacco (370 cases and 1,358 controls) by pooling individual level data of never smokers from all available studies; the associations between cannabis and lung cancer risk was then assessed with unconditional logistic regression adjusting for age, sex, highest education, race and study.
The shapes of nonlinear dose-response associations between the continuous exposure variables (joint equivalent per day, duration and overall joint-years) and lung cancer were examined using restricted cubic spline regression with 5 knots, adjusting for age, sex, race, highest education, tobacco smoking status (never vs. ever) and pack-years, as well as study.27 Study-specific multivariable unconditional logistic regression and pooled analysis for never smokers were conducted in SAS, while the pooled effect estimates and restricted cubic spline analyses were done in R package.
Results
The characteristics of the 6 participating case-control studies in ILCCO are summarized in Table 1. Except for one family-based study, the remaining studies include 2 that used population-based controls and 3 that used hospital or clinical-based controls that were frequency matched to cases on at least age and sex. Four studies were conducted in North America (3 in the US and 1 in Canada), while the remaining 2 studies were set in the UK and New Zealand. In all studies, cannabis consumption was assessed using self-reported levels on standard questionnaires or surveys. In total, 2,159 lung cancer cases and 2,985 controls were included in the analysis. The frequency distributions of demographic, histology and lung cancer risk factors for both the total population and never tobacco smokers are summarized in Table 2. Although the average age of controls was slightly younger than cases (median age was 53.0 ± 10.4 for controls vs. 57.3 ± 10.5 for cases), subjects were comparable with respect to sex and race distributions, with over 75% of subjects being White/Caucasian. As expected, controls were less likely than cases to have ever smoked tobacco. Squamous cell carcinoma (n = 394), adenocarcinoma (n = 913) and small cell lung cancer (n = 273) histologic subtypes make up over 70% of all cases in the study sample (Table 2).
Table 1. Characteristics of participating studies in the pooled analysis of Cannabis smoking and lung cancer risk.
| Continent and study | Principal investigator(s) |
Control source (matching factor) |
Study period | Location | Cannablis consumption | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lead question(s) | Age start |
Age quit |
Intensity | Joint-years | Cases | Controls | |||||
| North America | |||||||||||
| University of California, Los Angeles (UCLA)16 | Z.F. Zhang H. Morgenstern | Population-based (age, sex, neighborhood) | 1999-2004 | Los Angeles County, California, US | Have you ever smoked marijuana? Have you ever smoked hashish or hash oil? | • | • | • | • | 610 | 1,037 |
| Memorial Sloan-Kettering Cancer Center (MSKCC) | I. Orlow | Hospital-based (age, residence) | 2003-2006 | New York City, US | Do you or have you ever used marijuana? | • | • | • | 94 | 87 | |
| Mount Sinai Hospital-Princess Margaret Hospital Study (MSH-PMH)28 | R.J. Hung, G. Liu | Clinic-based (age, sex, residence) | 2008-2013 | Ontario, Canada | Have you ever used or tried marijuana, cannabis, or hashish? | • | • | • | • | 431 | 308 |
| Moffitt Cancer Study29 | P. Lazarus | Hospital-based (sex, race, residence) | 1999-2003 | Florida, US | Have you ever used marijuana at least once a day for one year? | • | • | • | • | 497 | 897 |
| Europe | |||||||||||
| ReSoLuCENT30 | M.D. Teare P.J. Woll | Family and clinic-based (race/residence) | 2006-2009 | North Trent, UK | Have you ever used any other tobacco-related products (such as chewing tobacco, cannabis, snuff, etc)? | • | • | 449 | 332 | ||
| Oceania | |||||||||||
| New Zealand15 | B. Cox | Population-based (age, sex, race) | 2001-2005 | New Zealand | Have you smoked more than 20 joints (of marijuana) (or the equivalent) in your lifetime? | • | • | • | • | 78 | 324 |
| Total | 2,159 | 2,985 | |||||||||
Abbreviations: US, United States; UK, United Kingdom;
data element collected.
Table 2. Frequency distribution of demographic characteristics, histology and smoking variables.
| All subjects | Never tobacco smokers | |||||||
|---|---|---|---|---|---|---|---|---|
| Cases (n = 2,159) | Controls (n = 2,985) | Cases (n = 370) | Controls (n = 1,358) | |||||
| No. | (%) | No. | (%) | No. | (%) | No. | (%) | |
| Age1 groups, years | ||||||||
| <50 | 366 | (17) | 956 | (32) | 88 | (24) | 492 | (36) |
| 50–59 | 985 | (46) | 1327 | (44) | 130 | (35) | 570 | (42) |
| 60–69 | 431 | (20) | 522 | (17) | 74 | (20) | 216 | (16) |
| ≥70 | 377 | (17) | 180 | (6) | 78 | (21) | 80 | (6) |
| Sex | ||||||||
| Male | 1080 | (50) | 1572 | (53) | 98 | (26) | 635 | (47) |
| Female | 1079 | (50) | 1413 | (47) | 272 | (74) | 723 | (53) |
| Race | ||||||||
| White/Caucasian | 1723 | (80) | 2323 | (78) | 222 | (60) | 1040 | (77) |
| Black/African American | 150 | (7) | 255 | (9) | 23 | (6) | 115 | (8) |
| Other | 286 | (13) | 407 | (14) | 125 | (34) | 203 | (15) |
| Education level | ||||||||
| Low (elementary school) | 373 | (17) | 195 | (7) | 45 | (12) | 62 | (5) |
| Medium (up to high school completion) | 836 | (39) | 977 | (33) | 119 | (32) | 399 | (29) |
| High (postsecondary education or higher) | 950 | (44) | 1813 | (61) | 206 | (56) | 897 | (66) |
| Smoking Status | ||||||||
| Never smoker | 370 | (17) | 1358 | (46) | 370 | (100) | 1358 | (100) |
| Ever smoker | ||||||||
| Former smoker | 678 | (31) | 938 | (32) | ||||
| Current smoker | 1089 | (50) | 663 | (22) | ||||
| Histology | ||||||||
| Squamous cell carcinoma | 394 | (18) | 21 | (6) | ||||
| Adenocarcinoma | 913 | (42) | 226 | (61) | ||||
| Small cell carcinoma | 273 | (13) | 9 | (2) | ||||
| Other | 579 | (27) | 114 | (31) | ||||
Age at diagnosis for cases and age at interview for controls.
Abbreviation: No.: number.
The frequency distribution of cannabis smoking variables for the overall population and never tobacco smokers as well as the pooled risk estimates are summarized in Table 3. The estimated ORs of all lung cancer associated with habitual cannabis smokers as compared to nonhabitual or never cannabis smokers in the participating studies ranged from 0.57 (95% CI: 0.30–1.07) in the Mount Sinai Hospital-Princess Margaret Hospital (MSH-PMH) study to 2.17 (95% CI: 1.04–4.52) in the New Zealand study (Fig. 1). The overall summary OR was 0.96 (95% CI: 0.66–1.38, p for heterogeneity: 0.17) (Table 3), and there was no detectable heterogeneity in the effect estimates for studies that used population-based controls vs. hospital or clinic-based controls (p = 0.24).
Table 3. Odds-ratio estimates for the association between Cannabis smoking characteristics and lung cancer, results for all lung cancer.
| Cannabis smoking characteristics | All subjects | Never tobacco smokers | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases (n = 2,159) | Controls (n = 2,985) | No. of studies | Pooled OR1 (95% Cl) | Het (P) | I2 | Cases (n = 370) | Controls (n = 1,358) | OR (95% CI)2 | |||||
| No. | % | No. | % | No. | % | No. | % | ||||||
| Status3 | |||||||||||||
| Nonhabitual smoker4 | 1,934 | (90) | 2,664 | (89) | 1.00 (Ref) | 359 | (97.0) | 1294 | (95.3) | 1.00 | |||
| Habitual | 225 | (10) | 321 | (11) | 6 | 0.96 (0.66-1.38) | 0.17 | 35.38 | 11 | (3.0) | 64 | (4.7) | 1.03 (0.51-2.08) |
| Intensity (joints/day)3 | |||||||||||||
| Nonhabitual smoker4 | 1,934 | (90) | 2,664 | (89) | 1.00 (Ref) | 359 | (97.0) | 1294 | (95.3) | 1.00 | |||
| <1 | 96 | (4) | 178 | (6) | 5 | 0.77 (0.51-1.16) | 0.32 | 15.51 | 9 | (2.4) | 41 | (3.0) | 1.33 (0.61-2.93) |
| ≥1 | 126 | (6) | 133 | (5) | 4 | 0.88(0.63-1.24)5 | 0.86 | 0.00 | 2 | (0.5) | 23 | (1.7) | 0.49 (0.11-2.25) |
| Continuous | 6 | 1.02 (0.92-1.13) | 0.28 | 20.82 | 1.08 (0.91-1.30) | ||||||||
| Duration, years3 | |||||||||||||
| Nonhabitual smoker4 | 1,934 | (90) | 2,664 | (89) | 1.00 (Ref) | 359 | (97.0) | 1294 | (95.3) | 1.00 | |||
| >0-<20 | 149 | (7) | 226 | (8) | 6 | 0.94 (0.70-1.26) | 0.40 | 2.35 | 8 | (2.2) | 49 | (3.6) | 0.89 (0.39-2.00) |
| ≥20 | 76 | (3) | 95 | (3) | 5 | 1.03 (054-1.98) | 0.07 | 53.21 | 3 | (0.8) | 15 | (1.1) | 1.64 (0.45-6.00) |
| Continuous | 3 | 0.99 (0.97-1.02)6 | 0.27 | 23.01 | 0.97 (0.93-1.01) | ||||||||
| Joint-years3 | |||||||||||||
| Nonhabitual smoker (<1)4 | 1,934 | (90) | 2,664 | (90) | 1.00 (Ref) | 359 | (97.0) | 1294 | (95.3) | 1.00 | |||
| 1-<10 | 94 | (4) | 180 | (6) | 5 | 0.69 (0.41-1.17) | 0.21 | 31.95 | 9 | (2.4) | 44 | (3.2) | 1.26 (0.57-2.75) |
| ≥10 | 128 | (6) | 132 | (4) | 4 | 0.94(0.67-1.32)7 | 0.62 | 0.00 | 2 | (0.5) | 20 | (1.5) | 0.54 (0.12-2.55) |
| Continuous | 5 | 1.00(0.99-1.00)8 | 0.61 | 0.00 | 1.00 (0.93-1.07) | ||||||||
| Age of start, years3 | |||||||||||||
| Nonhabitual smoker4 | 1,934 | (90) | 2,664 | (90) | 1.00 (Ref) | 359 | (97.0) | 1294 | (95.3) | 1.00 | |||
| >18 | 86 | (4) | 127 | (4) | 4 | 0.75 (0.53-1.08) | 0.45 | 0.00 | 6 | (1.6) | 28 | (2.1) | 1.25 (0.47-3.29) |
| ≤18 | 124 | (6) | 187 | (6) | 3 | 0.86(0.62-1.19)9 | 0.67 | 0.00 | 5 | (1.4) | 36 | (2.6) | 0.85 (0.32-2.31) |
Random effect model.
Adjusted for age, sex, race, highest education, and study.
Study-specific models were adjusted for age, sex, race, highest education, tobacco smoking status and tobacco smoking pack-years. Exceptions are models for continuous variables for Moffitt Cancer Study and Memorial Sloan-Kettering Cancer Center Study, which were adjusted for age, sex, and tobacco smoking pack-years as the base.
Nonhabitual cannabis smoker is defined as those with cumulative cannabis consumption of less than 1 joint-year (equivalent of 1 joint per day for 1 year), including never users.
Summary OR based on 4 studies excluding New Zealand (OR=7.16, 95%CI 2.07-24.78), P-value for heterogeneity when it is included = 0.03.
Summary OR based on 3 studies excluding Moffitt Cancer Study (OR=1.16,95%CI 1.01-1.33), MSH-PMH Study (OR=0.97, 95%CI 0.97-0.99) and New Zealand (OR=1.05, 95%CI 1.01-1.09), P-value for heterogeneity when they are included < 0.001.
Summary OR based on 4 studies excluding New Zealand (OR=7.39, 95%CI 2.37-23.04), P-value for heterogeneity when it is included = 0.01.
Summary OR based on 5 studies excluding New Zealand (OR=1.09, 95%CI 1.03-1.16), P-value for heterogeneity when it is included = 0.03.
Summary OR based on 3 studies excluding New Zealand (OR=0.93, 95%CI 0.79-1.09), P-value for heterogeneity when it is included = 0.02.
Abbreviations: CI, confidence interval; Het, heterogeneity; No., number.
Figure 1.
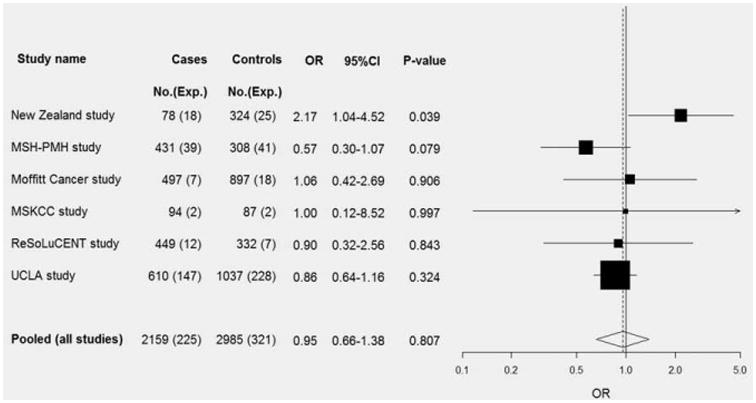
Forest plot of the association between cannabis smoking (habitual vs. nonhabitual) and lung cancer risk. Pooled, pooled OR according to a random effects model (p-heterogeneity = 0.17). Abbreviations: No.: Number; Exp.: Number exposed; CI: confidence interval. MSH-PMH study, The Mount Sinai Hospital-Princess Margaret Hospital Study; MSKCC study, The Memorial Sloan-Kettering Cancer Center Study; UCLA study, The University of California at Los Angeles Study.
Compared to nonhabitual or never users, the pooled OR was 0.88 (95% CI: 0.63–1.24) for individuals who smoked 1 or more joint-equivalents per day and 1.03 (95%CI: 0.54–1.98) for individuals who smoked cannabis for 20 years or more (Table 3) for all lung cancer cases. Also, compared to nonhabitual or never users, the OR for those who started smoking cannabis before 19 years of age was 0.86 (95% CI: 0.62–1.19).
In general, sex-specific analyses yielded results comparable to those obtained for all lung cancer cases and were therefore not presented in detail. For example, compared to nonhabitual or never cannabis users, the ORs for habitual users are 0.84 (95%CI: 0.60, 1.18) for men and 0.94 (95%CI: 0.62, 1.40) for women. The OR for those who smoked cannabis more than 20 years was 0.80 (95%CI: 0.55, 1.16) for men and 1.05 (95%CI: 0.48, 2.82) for women.
No overall association between cannabis smoking and all lung cancer was detected among never tobacco smokers; habitual versus nonhabitual or never user OR was 1.03 (95% CI: 0.51–2.08). Effect estimates for the other categorical cannabis variables were too imprecise to be informative due to the small numbers of exposed cases. For example, the OR was 1.64 (95%CI: 0.45–6.00) for those who smoked 20 years or more versus those nonhabitual or never smokers (Table 3), and it was 0.54 (95% CI: 0.12–2.55) for 10 or more joint-years of cannabis use versus less than 1 joint-year. The majority of the never tobacco-smoking lung-cancer cases are female (272 out of a total of 370), and restricting the analysis to female nontobacco smokers yielded comparable results: Compared to nonhabitual or never users, the OR was 0.94 (95%CI: 0.62, 1.40) for habitual cannabis users and 1.05 (95%CI: 0.48, 2.28) for those who smoked for 20 years or more.
The results for the largest histologic subgroup, adenocarcinoma, are presented in Table 4. There was a suggestive association between high intensity and cumulative cannabis smoking on adenocarcinoma lung cancer. Compared to non-habitual or never users, the OR for users who smoked one or more joint-equivalents per day was 1.73 (95% CI: 0.75–4.00), and OR for those with cumulative exposure of 10 joint-years or more was 1.74 (95% CI: 0.85–3.55) (Table 4). Nonetheless, we observed little association with the duration of cannabis smoking; the OR was 1.08 (95% CI: 0.60–1.96) for those who smoked for 20 years or more. Only 4 of the studies have sufficient data for squamous cell carcinoma. Compared to non-habitual or never users, the estimated OR for squamous cell carcinoma was 1.55 (95%CI: 0.35–6.87) for those who smoked one or more joint-equivalents per day and 1.58 (95%CI: 0.48–5.20) for smokers of more than 20 years. The estimated OR for the association between cumulative exposure of 10 or more joint-years (vs. less than 1 joint-year) and lung cancer was 2.35 (95%CI: 0.48–11.46). The confidence intervals were very wide due to smaller sample sizes of squamous cell carcinoma.
Table 4. Risk estimates for the association between Cannabis smoking characteristics and lung cancer risk, results for adenocarcinoma.
| Cannabis smoking characteristics | Adenocarcinoma cases only | |||||||
|---|---|---|---|---|---|---|---|---|
| Cases (n = 913) | Controls (n = 2,985) | No of studies | Pooled OR1 (95% CI) | Het (P) | I2 | |||
| No. | % | No. | % | |||||
| Status1 | ||||||||
| Nonhabitual smoker2 | 812 | (89) | 2664 | (89) | 1.00 (Ref) | |||
| Habitual | 101 | (11) | 321 | (11) | 6 | 0.99 (0.73–1.33) | 0.58 | 0.00 |
| Intensity (joints/day)1 | ||||||||
| Nonhabitual smoker2 | 812 | (89) | 2664 | (90) | 1.00 (Ref) | |||
| <1 | 41 | (5) | 178 | (6) | 5 | 0.72 (0.48–1.10) | 0.71 | 0.00 |
| ≥1 | 58 | (6) | 133 | (4) | 5 | 1.73 (0.75–4.00) | 0.05 | 57.87 |
| Continuous | 5 | 1.04 (0.93–1.17) | 0.27 | 21.88 | ||||
| Duration, years1 | ||||||||
| Nonhabitual smoker2 | 812 | (89) | 2664 | (89) | 1.00 (Ref) | |||
| >0 to <20 | 62 | (7) | 226 | (8) | 6 | 0.98 (0.69–1.39) | 0.92 | 0.00 |
| ≥20 | 39 | (4) | 95 | (3) | 5 | 1.08 (0.60–1.96) | 0.27 | 22.92 |
| Continuous | 6 | 0.99 (0.97–1.02) | 0.12 | 43.05 | ||||
| Joint-years1 | ||||||||
| Nonhabitual smoker (<1)1 | 812 | (89) | 2664 | (90) | 1.00 (Ref) | |||
| 1 to <10 | 37 | (4) | 180 | (6) | 5 | 0.67 (0.41–1.11) | 0.36 | 7.44 |
| ≥10 | 62 | (7) | 132 | (4) | 5 | 1.74 (0.85–3.56) | 0.09 | 50.34 |
| Continuous | 5 | 1.00 (0.99–1.00)3 | 0.97 | 0.00 | ||||
Random effect model (Study-specific models adjusted for age, sex, race, highest education, tobacco smoking status and tobacco smoking pack-years. Exceptions are models for continuous variables for Moffitt Cancer Study and Memorial Sloan-Kettering Cancer Center Study, which were adjusted for age, sex and tobacco smoking pack-years as the base.
Nonhabitual cannabis smoker is defined as ones with cumulative cannabis consumption of less than 1 joint-year (equivalent of 1 joint per day for 1 year), including never users.
Summary OR based on 5 studies excluding New Zealand (OR = 1.11, 95%CI 1.04–1.19), p-value for heterogeneity when it is included = 0.04.
Abbreviations: CI, confidence interval; Het, heterogeneity; No., number.
Use of restricted cubic splines to examine the dose-response associations between cannabis use and lung-cancer incidence did not exhibit monotonic associations for average joints per day or duration of use (Figs. 2a and 2b). There was, however, a positive monotonic association between joint-years of cannabis use and lung cancer (Fig. 2c); but the 95% confidence bands were wide, especially for higher exposure levels.
Figure 2.
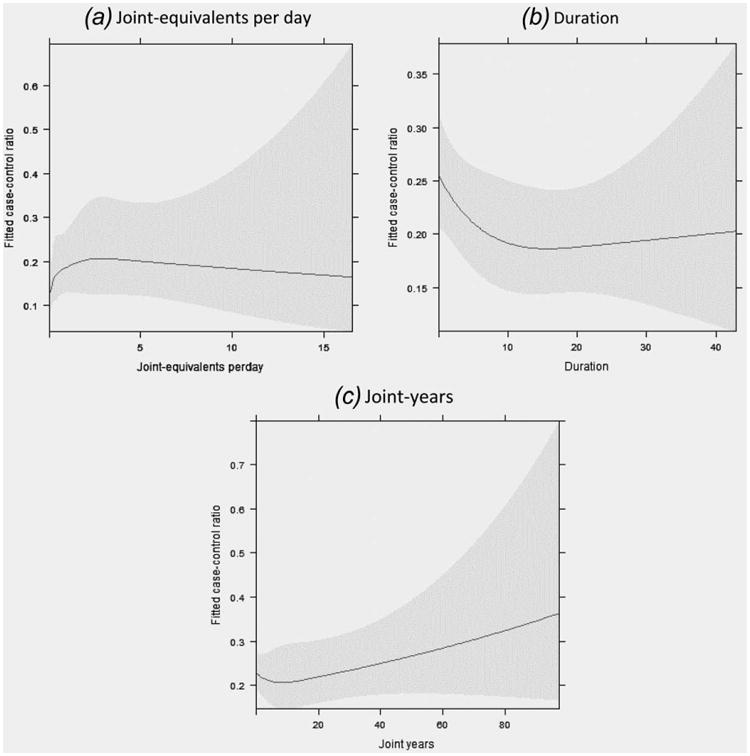
Restricted cubic spline (5 knots) to explore nonlinear association between cannabis smoking and lung cancer risk in all subjects showing the fitted odds of being a case versus being a control under different exposure measurements: (a) Joint-equivalents per day; (b) Duration; (c) Joint-years. Gray area, 95% confidence interval. Each model was adjusted for age, sex, race, highest education, tobacco smoking status and packyears, and study.
Discussion
In this study, we harmonized and pooled individual-level data from 6 ILCCO studies and examined the association with lung cancer for cannabis smoking status, age of start, intensity, duration and cumulative exposure in all subjects and subgroups by sex and histologic subtype where sample size allowed, and also separately for never tobacco smokers. In our pooled results, we found little or no association between the intensity, duration, cumulative consumption or age of start of cannabis smoke and the risk of lung cancer in all subjects or never smokers, and suggestive association for adenocarcinoma. The evidence for the association with other histological subtypes is limited by the small sample size. In the spline analyses, there was a weak increasing trend over long-term and high levels of cumulative cannabis smoking exposure. The confidence intervals were wide due to the limited number of observations at the high exposure levels, but the results are more compatible with an association with lung cancer at high levels of cannabis exposure than with no association. In addition, misclassification of cannabis use no doubt occurred and may have flattened or distorted the dose-response relation.
The consumption of psychoactive substances such as cannabis and tobacco are often highly correlated. Therefore, given the established link between tobacco smoking and lung cancer, confounding by tobacco smoking is one of the major concerns in studies attempting to elucidate the association between cannabis smoking and lung cancer. In this study, we found habitual cannabis users were much more likely than nonhabitual or never users to be tobacco smokers (86.3% vs. 64.0%, p < 0.001). Similarly, tobacco smokers were more likely than never smokers to use cannabis habitually (13.8% vs. 4.3%, p < 0.001). Comparing cannabis users who also smoked tobacco versus those who used cannabis alone, users of both products were more likely to be males and with low to medium education levels (data not shown). We expect that the association of cannabis and tobacco smoking would lead to upward confounding of the cannabis association with lung cancer, and that misclassification of cigarette consumption would be a source of overestimating the strength of the cannabis-lung cancer association in our data.
One of the key advantages of this pooled analysis was the examination of the association between cannabis smoking and lung cancer among never tobacco smokers, and these findings were consistent with the results in the total sample. The use of random-effect models in the pooled analysis reduced the likelihood of larger studies overly influencing the effect estimates. Since all studies in this pooled analysis were case-control studies, and medical cannabis may have been used by some to alleviate pain caused by cancer or pre-cancer symptoms, there is possible reverse causality. However, our results did not change after excluding any cannabis exposures within 2 years prior to the date of diagnosis or interview (data not shown), arguing against reverse causality.
Our pooled results are consistent with a previous systematic review of observational studies on the association between marijuana smoking and lung cancer, which also found no association after adjusting for tobacco use.31 However, in a pooled analysis of three studies conducted in Maghreb restricted to men, the OR was 2.3 (95% CI: 1.5–3.6) for ever cannabis smoking comparing lung cancer cases to hospital-based controls, after adjusting for tobacco smoking and other potential confounders.17 The investigators of that study also observed an increased risk of lung cancer with increasing joint-years.17 But as the authors pointed out, the practice of mixing of tobacco with cannabis in the region would lead to upward residual confounding by tobacco smoking. In a recently published 40-year cohort study among Swedish military conscripts, a positive association was observed between heavy cannabis smoking (defined as more than 50 times in total) at baseline and lung–cancer incidence32; however, there are important concerns regarding potential reporting bias, association of tobacco and cannabis smoking behaviors (especially changes in such behaviors since cohort entry), and residual confounding by tobacco smoking, all of which may have affected the validity of the findings.
The dose of cannabis product consumption differs by source of plant material, its processing, and by smoking techniques including the depth of inhalation and breath-holding, number and frequency of puffs, as well as how much of the joint is smoked. In addition, the amount of combustion byproduct varies by the mode of consumption. Because of the varied levels of information collected by individual studies, we were unable to examine these effects in this pooled analysis. We observed interstudy heterogeneity, and it is possible that this heterogeneity is due to differing characteristics of the individual studies such as the prevailing cannabis product being consumed in the study region, and differential recall bias between in-person versus self-administered questionnaire formats. Because of the limited number of studies, we were unable to fully explore such sources of heterogeneity using meta-regression. However, we detected no difference in the pooled ORs between population-based case-control studies and hospital or clinic-based studies. The study that contributed most of the observed heterogeneity is the New Zealand study, which is also the study that reported the strongest association between cannabis smoking and lung cancer risk. The exclusion of this study resulted in the ORs closer to null, with a much reduced heterogeneity.
Cannabis use is under international control and its legal status varies, so reporting bias is of concern. The reported prevalence among controls in the study populations is comparable to nation or region-specific survey results.33–35 Greater under-reporting by cases as compared to controls might explain the lack of observed association.
Cannabis is usually smoked without a filter, and smoking dynamics studies among habitual marijuana users show that the overall burden of particulates delivered to the respiratory tract is about 4 times greater when smoking marijuana than when smoking the same amount of tobacco.8 When compared to tobacco smoke, cannabis tends to burn at a higher temperature (which may accelerate biochemical processes) and cannabis smoke is typically inhaled deeper and held longer.36 On the other hand, the quantity of cannabis smoked by chronic users is typically far less than the quantity of tobacco smoked by chronic cigarette smokers.
Other potential health effects of cannabis smoking that have been reported include those on the cardiovascular system,37–39 the development of drug dependence, and subtle cognitive impairment. Inconclusive findings of cannabis use being associated with mild changes in pulmonary function40–44 have also been reported, as have its benefits on fasting glucose.45 The changing social and legal status of cannabis in the United States may however complicate the picture by altering patterns of consumption methods. Specifically, respiratory risks may differ with the use of water pipes and vaporizers or with consuming oral preparations.
Given the popularity of cannabis use, particularly among younger populations, it is important to have reliable estimates of health consequences. Our study highlights the need for comprehensive and standardized measures of quantity and methods of cannabis consumption, along with accurate measures of tobacco consumption to insure confounding control. To address the small sample size with very high exposures and histological subtypes (other than adenocarcinoma), future epidemiologic studies need to include sufficient numbers of heavy users and specific histological subtypes of interests. Prospective follow-up studies of the health effects are also needed.
What's new?
Due to the potential adverse effect of cannabis smoking and its popularity, an investigation of its association with lung cancer risk is essential to help support appropriate regulations as well as health and social policy responses. The analysis presented here included the largest data set on cannabis and lung cancer risk to date. Its non-linear dose-response was examined using restricted cubic spline regression, a first in this line of work. Results provide little evidence for an increased risk of lung cancer among habitual or long-term cannabis smokers, although the possibility of potential adverse effect for heavy consumption cannot be excluded.
Acknowledgments
Grant sponsor: National Institutes of Health; Grant numbers: DA 11386, ES011667, CA 90833, and CA 09142; Grant sponsor: Canadian Cancer Society Research Institute; Grant number: 020214; Grant sponsor: USPHS; Grant number: P01-CA68384, R01-DE13158; Grant sponsors: Alper Research funds, Sheffield Experimental Cancer Medicine Centre and Weston Park Hospital Cancer Charity; Steps for Breath, the Labrecque Foundation, and the Society of Memorial Sloan-Kettering Cancer Center
References
- 1.UNODC. World Drug Report. 2012 [Google Scholar]
- 2.Administration SAaMHS. World Drug Report 2011. 2011 [Google Scholar]
- 3.Adami HO, Hunter D, Trichopoulos D. Textbook of cancer epidemiology. 2. New York, NY: Oxford University Press; 2008. [Google Scholar]
- 4.Schottenfeld D, F JF., Jr . Cancer epidemiology and prevention. 3rd. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 5.Humans IWGotEoCRt. World Health Organization, International Agency for Research on Cancer. 2004. Tobacco Smoke and Involuntary Smoking. [Google Scholar]
- 6.Moir D, Rickert WS, Levasseur G, et al. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem Res Toxicol. 2008;21:494–502. doi: 10.1021/tx700275p. [DOI] [PubMed] [Google Scholar]
- 7.Denissenko MF, Pao A, Tang M, et al. Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hotspots in P53. Science. 1996;274:430–2. doi: 10.1126/science.274.5286.430. [DOI] [PubMed] [Google Scholar]
- 8.Wu TC, Tashkin DP, Djahed B, et al. Pulmonary hazards of smoking marijuana as compared with tobacco. N Engl J Med. 1988;318:347–51. doi: 10.1056/NEJM198802113180603. [DOI] [PubMed] [Google Scholar]
- 9.Fligiel SE, Roth MD, Kleerup EC, et al. Tracheo-bronchial histopathology in habitual smokers of cocaine, marijuana, and/or tobacco. Chest. 1997;112:319–26. doi: 10.1378/chest.112.2.319. [DOI] [PubMed] [Google Scholar]
- 10.Gong H, Jr, Fligiel S, Tashkin DP, et al. Tracheo-bronchial changes in habitual, heavy smokers of marijuana with and without tobacco. Am Rev Respir Dis. 1987;136:142–9. doi: 10.1164/ajrccm/136.1.142. [DOI] [PubMed] [Google Scholar]
- 11.Barsky SH, Roth MD, Kleerup EC, et al. Histopatho-logic and molecular alterations in bronchial epithelium in habitual smokers of marijuana, cocaine, and/or tobacco. J Natl Cancer Inst. 1998;90:1198–205. doi: 10.1093/jnci/90.16.1198. [DOI] [PubMed] [Google Scholar]
- 12.Busch FW, Seid DA, Wei ET. Mutagenic activity of marihuana smoke condensates. Cancer Lett. 1979;6:319–24. doi: 10.1016/s0304-3835(79)80088-9. [DOI] [PubMed] [Google Scholar]
- 13.Roy PE, Magnan-Lapointe F, Huy ND, et al. Chronic inhalation of marijuana and tobacco in dogs: pulmonary pathology. Res Commun Chem Pathol Pharmacol. 1976;14:305–17. [PubMed] [Google Scholar]
- 14.Zhu LX, Sharma S, Stolina M, et al. Delta-9-tetrahydrocannabinol inhibits antitumor immunity by a CB2 receptor-mediated, cytokine-dependent pathway. J Immunol. 2000;165:373–80. doi: 10.4049/jimmunol.165.1.373. [DOI] [PubMed] [Google Scholar]
- 15.Aldington S, Harwood M, Cox B, et al. Cannabis use and risk of lung cancer: a case-control study. Eur Respir J. 2008;31:280–6. doi: 10.1183/09031936.00065707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashibe M, Morgenstern H, Cui Y, et al. Marijuana use and the risk of lung and upper aerodigestive tract cancers: results of a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2006;15:1829–34. doi: 10.1158/1055-9965.EPI-06-0330. [DOI] [PubMed] [Google Scholar]
- 17.Berthiller J, Straif K, Boniol M, et al. Cannabis smoking and risk of lung cancer in men: a pooled analysis of three studies in Maghreb. J Thorac Oncol. 2008;3:1398–403. doi: 10.1097/JTO.0b013e31818ddcde. [DOI] [PubMed] [Google Scholar]
- 18.Sasco AJ, Merrill RM, Dari I, et al. A case-control study of lung cancer in Casablanca, Morocco. Cancer Causes Control. 2002;13:609–16. doi: 10.1023/a:1019504210176. [DOI] [PubMed] [Google Scholar]
- 19.Sidney S, Quesenberry CP, Jr, Friedman GD, et al. Marijuana use and cancer incidence (California, United States) Cancer Causes Control. 1997;8:722–8. doi: 10.1023/a:1018427320658. [DOI] [PubMed] [Google Scholar]
- 20.Voirin N, Berthiller J, Benhaim-Luzon V, et al. Risk of lung cancer and past use of cannabis in Tunisia. J Thorac Oncol. 2006;1:577–9. [PubMed] [Google Scholar]
- 21.Sridhar KS, Raub WA, Jr, Weatherby NL, et al. Possible role of marijuana smoking as a carcinogen in the development of lung cancer at a young age. J Psychoactive Drugs. 1994;26:285–8. doi: 10.1080/02791072.1994.10472442. [DOI] [PubMed] [Google Scholar]
- 22.Taylor FM., III Marijuana as a potential respiratory tract carcinogen: a retrospective analysis of a community hospital population. South Med J. 1988;81:1213–6. doi: 10.1097/00007611-198810000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Hung RJ, Christiani DC, Risch A, et al. International Lung Cancer Consortium: pooled analysis of sequence variants in DNA repair and cell cycle pathways. Cancer Epidemiol Biomarkers Prev. 2008;17:3081–9. doi: 10.1158/1055-9965.EPI-08-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Programme on Substance Abuse WHODoMHaPoSA. Cannabis: a health perspective and research agenda. Division of Mental Health and Prevention of Substance Abuse, World Health Organization; 1997. [Google Scholar]
- 25.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galbraith R. Graphical display of estimates having different standard errors. Technometrics. 1988;30:271–81. [Google Scholar]
- 27.Rothman KJ, Greenland S. Modern epidemiology. 2nd. Philadelphia, PA: Lippincott-Raven; 1998. [Google Scholar]
- 28.Wang Y, McKay JD, Rafnar T, et al. Imputation from the 1000 Genomes Project identifies rare large effect vairants of BRCA2-K3326X and CHECK2-I157T as risk factors for lung cancer. Nat Genet. in press. [Google Scholar]
- 29.Gallagher CJ, Muscat JE, Hicks AN, et al. The UDP-glucuronosyltransferase 2B17 gene deletion polymorphism: sex-specific association with urinary 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol glucuronidation phenotype and risk for lung cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:823–8. doi: 10.1158/1055-9965.EPI-06-0823. [DOI] [PubMed] [Google Scholar]
- 30.ReSoLuCENT PJW. Resource for the Study of Lung Cancer Epidemiology in North Trent. 2009;2013:45. [Google Scholar]
- 31.Mehra R, Moore BA, Crothers K, et al. The association between marijuana smoking and lung cancer: a systematic review. Arch Intern Med. 2006;166:1359–67. doi: 10.1001/archinte.166.13.1359. [DOI] [PubMed] [Google Scholar]
- 32.Callaghan RC, Allebeck PA, Sidorchuk A. Marijuana use and risk of lung cancer: a 40-year cohort study. Cancer Causes Control. 2013;24:1811–1820. doi: 10.1007/s10552-013-0259-0. [DOI] [PubMed] [Google Scholar]
- 33.Addiction EMCfDaD. European Monitoring Centre for Drugs and Drug Addiction Annual Report 2012–The State of the Drugs Problem in Europe. European Monitoring Centre for Drugs and Drug Addiction; 2012. [Google Scholar]
- 34.Degenhardt L, Chiu WT, Sampson N, et al. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: findings from the WHO World Mental Health Surveys. PLoS Med. 2008;5:e141. doi: 10.1371/journal.pmed.0050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ialomiteanu AR, Adlaf EM, Mann RE, et al. CAMH Monitor eReport: addiction and mental health indicators among ontario adults, 1977–2009. Centre for Addiction and Mental Health; 2011. [Google Scholar]
- 36.Council on Scientific Affairs. Marijuana: its health hazards and therapeutic potentials. J Am Med Assoc. 1981;246:1823–7. [PubMed] [Google Scholar]
- 37.Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol. 2002;42:58S–63S. doi: 10.1002/j.1552-4604.2002.tb06004.x. [DOI] [PubMed] [Google Scholar]
- 38.Mittleman MA, Lewis RA, Maclure M, et al. Triggering myocardial infarction by marijuana. Circulation. 2001;103:2805–9. doi: 10.1161/01.cir.103.23.2805. [DOI] [PubMed] [Google Scholar]
- 39.Mukamal KJ, Maclure M, Muller JE, et al. An exploratory prospective study of marijuana use and mortality following acute myocardial infarction. Am Heart J. 2008;155:465–70. doi: 10.1016/j.ahj.2007.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aldington S, Williams M, Nowitz M, et al. Effects of cannabis on pulmonary structure, function and symptoms. Thorax. 2007;62:1058–63. doi: 10.1136/thx.2006.077081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hancox RJ, Poulton R, Ely M, et al. Effects of cannabis on lung function: a population-based cohort study. Eur Respir J. 2010;35:42–7. doi: 10.1183/09031936.00065009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pletcher MJ, Vittinghoff E, Kalhan R, et al. Association between marijuana exposure and pulmonary function over 20 years. J Am Med Assoc. 2012;307:173–81. doi: 10.1001/jama.2011.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tashkin DP, Coulson AH, Clark VA, et al. Respiratory symptoms and lung function in habitual heavy smokers of marijuana alone, smokers of marijuana and tobacco, smokers of tobacco alone, and non-smokers. Am Rev Respir Dis. 1987;135:209–16. doi: 10.1164/arrd.1987.135.1.209. [DOI] [PubMed] [Google Scholar]
- 44.Bloom JW, Kaltenborn WT, Paoletti P, et al. Respiratory effects of non-tobacco cigarettes. Br Med J (Clin Res Ed) 1987;295:1516–8. doi: 10.1136/bmj.295.6612.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Penner EA, Buettner H, Mittleman MA. The impact of marijuana use on glucose, insulin, and insulin resistance among US Adults. Am J Med. 2013;126:583–9. doi: 10.1016/j.amjmed.2013.03.002. [DOI] [PubMed] [Google Scholar]


