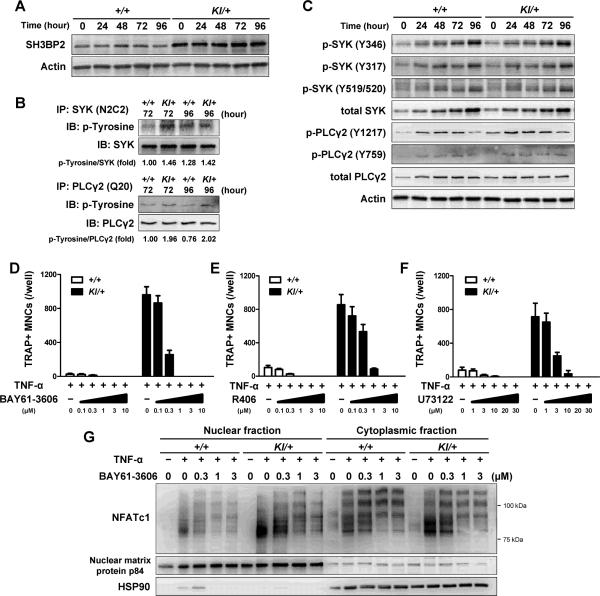
Fig. 4. P416R SH3BP2 cherubism mutation increases SYK and PLCγ2 phosphorylation during TNF-α-induced osteoclastogenesis.
Bone marrow cells were isolated from Sh3bp2+/+ and Sh3bp2KI/+ mice. After 2-day preculture with M-CSF (25 ng/ml), cells were stimulated with TNF-α (100 ng/ml). Protein samples were collected at the indicated time points. (A) Western blot analysis for SH3BP2 in Sh3bp2+/+ and Sh3bp2KI/+ BMMs stimulated with TNF-α. (B) Immunoprecipitation and Western blot analysis for the phosphorylation of SYK and PLCγ2. SYK and PLCγ2 proteins were immunoprecipitated using anti-SYK (N2C2) and anti-PLCγ2 (Q20) antibodies, respectively, and probed by anti-phosphotyrosine antibody (clone: 4G10). Intensities of bands were quantified, and ratio of phosphotyrosine to SYK or to PLCγ2 were calculated and normalized to that of Sh3bp2+/+ samples at 72 hours. (C) Western blot analysis for phosphorylated SYK and PLCγ2. (D–F) Quantitation of TRAP+ MNCs. BMMs were stimulated with TNF-α (100 ng/ml) for 96 hours in the presence of SYK inhibitors (BAY61-3606 (D) and R406 (E)), PLC inhibitor (U73122 (F)), and DMSO (as control). (G) NFATc1 protein levels in nuclear and cytoplasmic fractions. Protein samples were collected at 72 hours after TNF-α (100 ng/ml) treatment in the presence of BAY61-3606. Data are presented as mean ± SD. +/+: Sh3bp2+/+, KI/+: Sh3bp2KI/+.