Abstract
Preterm infants are challenged by immature infant behavioral organization which may negatively influence their ability to oral feed. The purpose of this study was to determine whether the integrated H-HOPE (Hospital to Home: Optimizing the Infant’s Environment) intervention would improve infant behavioral organization by increasing the frequency of orally directed behaviors and the proportion of time spent in an alert behavioral state when offered prior to oral feeding. Mother–infant dyads (n = 198) were randomly assigned to the H-HOPE intervention or the Attention Control groups. Infants were born at 29 to 34 weeks gestation and were clinically stable. Mothers had at least two social environmental risk factors such as minority status or less than high school education. H-HOPE is an integrated intervention that included (1) twice-daily infant directed stimulation using the ATVV intervention (auditory, tactile, visual, and vestibular stimuli) and (2) maternal participatory guidance sessions by a nurse-community advocate team. Orally directed behaviors and behavioral states were assessed weekly prior to feeding during hospitalization when infants were able to feed orally. There were no differences between the groups at baseline (Day 0, prior to the initiation of the integrated H-HOPE intervention). We observed a pattern of increased frequency of orally directed behaviors in the H-HOPE intervention group when compared to the Attention Control group, however, the proportion of time spent in an alert behavioral state remained stable in both groups over the course of the study. On Day 7, the H-HOPE intervention group exhibited a significantly higher mean frequency of orally directed behaviors than the Attention Control group (12.6 vs. 7.1 pre-intervention, 51.8 vs. 33.2 during intervention, 4.3 vs. 3.2 immediately after intervention, and 8.9 vs. 5.3 immediately prior to feeding). On Day 7, the H-HOPE intervention group exhibited a significantly higher proportion of time spent in an alert behavioral state only during intervention (0.26 vs. 0.11) and immediately after intervention (0.28 vs. 0.06). These findings are suggestive that the integrated H-HOPE intervention facilitated infant behavioral organization for clinically stable infants born between 29 and 34 weeks gestation. The orally directed behaviors appear to be an important indicator of the infant’s preparation for feeding, and when used in conjunction with assessment of behavioral states, are especially valuable to the clinician. Use of this combined assessment approach in practice would strengthen clinician assessment for initiation of (beginning the first oral feeding) and daily preparation for oral feeding in preterm infants.
Keywords: orally directed behaviors, behavioral states, multisensory intervention, preterm infants
Preterm infants are challenged by immature infant behavioral organization (Ardura, Andres, Aldana, & Revilla, 1995; Holditch-Davis, 1990; Ingersoll & Thoman, 1999; Thoman, 1990). Infant behavioral organization is thought to influence the infant’ ability to feed orally, especially when infants demonstrate orally directed behaviors prior to feeding (White-Traut, Berbaum, Lessen, McFarlin, & Cardenas, 2005; White-Traut et al., 2002b) and alert behavioral states prior to and during feeding (Lau, 2012; Medoff-Cooper, Bilker, & Kaplan, 2010; Pickler, Best, Reyna, Gutcher, & Wetzel, 2006; White-Traut et al., 2005; White-Traut et al., 2002a; White-Traut et al., 2002b). However, little is known regarding the occurrence of orally directed behaviors in preterm infants prior to oral feeding (White-Traut et al., 2005; White-Traut et al., 2002b). Prefeeding interventions have been evaluated, particularly in regard to facilitating an alert behavioral state in preterm infants prior to feeding (White-Traut et al., 2005; White-Traut et al., 2002a; White-Traut et al., 2002b; White-Traut, Nelson, Silvestri, Patel, & Kilgallon, 1993), yet less is known regarding how prefeeding interventions might increase the frequency of orally directed behaviors prior to oral feeding. The purpose of this study was to determine whether the integrated H-HOPE (Hospital to Home: Optimizing the Infant’s Environment) intervention for preterm infants would increase the frequency of orally directed behaviors and the proportion of time spent in an alert behavioral state when offered prior to oral feeding.
Orally Directed Behaviors
Orally directed behaviors communicate the infant’s readiness to feed (Bell, White-Traut, & Rankin, 2012; Cagan, 1995). These orally directed behaviors are often evaluated by the occurrence of several prefeeding cues, including mouthing, rooting, tonguing, hand-to-mouth, hand swipes at mouth, empty sucking, sucking on hand, and sucking on tongue (Cagan, 1995; White-Traut et al., 2005; White-Traut et al., 2002b). In full-term infants, orally directed behaviors often begin to emerge in the first hour after birth. After birth, mouthing and rooting occur within 3 to 21 minutes, sucking within 15 minutes, tonguing within 15–27 minutes, and hand to mouth activity occurs within 12–34 minutes (Matthiesen, Ransjo-Arvidson, Nissen, & Uvnas-Moberg, 2001; Ransjo-Arvidson et al., 2001; Widstrom et al., 2011).
Orally directed behaviors are critical indicators of organized oromotor neurobehaviors, reflective of the infant’s ability to self-comfort and regulate behavioral states (Bell, Lucas, & White-Traut, 2008). When an infant is more organized, transition from crying to calm occurs when s/he is able to maintain sucking on hand activity (Bell et al., 2012). Repeated episodes of swiping at mouth are considered a less organized behavior whereas a sustained hand to mouth is more difficult to achieve and is considered a more organized behavior (Bell et al., 2012). In full-term infants, high frequency of orally directed behaviors is associated with successful latch and sustained breastfeeding (Erlandsson, Dsilna, Fagerberg, & Christensson, 2007; Hentschel, Ruff, Juette, von Gontard, & Gortner, 2007; Ransjo-Arvidson et al., 2001).
Rooting stimulates the infant’s sensory and tactile systems, providing the infant with internal information and allowing preparation for food consumption (Lemons & Lemons, 1996; Panniers, 2002). Vigorously sucking, rooting, and crying have been selected by practitioners as the three most important factors that signify hunger in preterm infants (Kinneer & Beachy, 1994). However, preterm infants’ orally directed behaviors are less organized and more subtle than full-term infants’ cues. As a result, preterm infants do not consistently give clinicians and caregivers clear cues about hunger, distress, or wakefulness (Cagan, 1995; Foy et al., 1997; Geertsma, Hyams, Pelletier, & Reiter, 1985). For example, a term infant may cry loudly and suck on hand vigorously to alert his caregiver about hunger needs, whereas a preterm infant might suck lightly on a pacifier or be in a drowsy or an alert state prior to feeding (Riordan, Iwata, Finney, Wohl, & Stanley, 1984). Yet, in preterm infants, even these less clear orally directed behaviors immediately prior to feeding are significant predictors of feeding efficiency (intake calculated as ml/min) (White-Traut et al., 2005; White-Traut et al., 2002b).
Behavioral States
The development of sleeping and waking states throughout infancy reflect the development of the central nervous system (Ardura et al., 1995; Ingersoll & Thoman, 1999). Deviations from normal state development can indicate neurological pathology (Holditch-Davis, Edwards, & Helms, 1998; Holditch-Davis, Scher, Schwartz, & Hudson-Barr, 2004). In his early research, Wolff identified several distinct behavioral states including “alert-inactivity,” “alert-activity,” and “drowsy” states and noted that the percentage of time an infant spent in “alert-inactivity” increased steadily over the first month of life (Wolff, 1965). Common categorizations of behavioral states used by researchers include quiet sleep, active sleep, drowsiness, quiet alert, active alert, crying, and indeterminant state (Korner, 1972; Thoman, 1990; Wolff, 1966). In both preterm and full-term infants, a predictable pattern of state development has been observed which is characterized by a decrease in active sleep and an increase in quiet sleep, alert states, and sleep state organization (Foreman, Thomas, & Blackburn, 2008; Holditch-Davis, 1990; Holditch-Davis & Edwards, 1998a; Holditch-Davis et al., 2004). Differences in the frequency of the different behavioral states have been noted in infants born at 34, 37, and 40 weeks gestation (Mouradian, Als, & Coster, 2000). Immature development of cortical organization, particularly in the frontal and prefrontal regions controlling behavioral states may contribute to these observed differences (Duffy, Als, & McAnulty, 1990). Compared to full-term infants, preterm infants spend more time in transitional states and less time in alert/awake states (Holditch-Davis, 1990; Holditch-Davis & Edwards, 1998a; Holditch-Davis et al., 1998). Preterm infants have a limited ability to maintain an alert state (Panniers, 2002). However, this limited ability to maintain alertness is important for social interaction and is correlated with the ability to feed orally (Gill, Behnke, Conlon, & Anderson, 1992; Panniers, 2002; Pickler, Best, Reyna, Wetzel, & Gutcher, 2005; White-Traut et al., 2005; White-Traut et al., 2002b). Infants who are alert immediately prior to oral feeding have improved oral feeding efficiency.
Documented Effectiveness of ATVV Intervention
Aiming at facilitating the high proportion of time spent in an alert behavioral state, previous research has demonstrated the effectiveness of the ATVV intervention, which provides social interaction via auditory (human voice), tactile (moderate stroking or massage), and visual (eye-to-eye contact), and vestibular stimulation (horizontal rocking) (Burns, Cunningham, White-Traut, Silvestri, & Nelson, 1994; White-Traut et al., 2005; White-Traut et al., 2002a; White-Traut et al., 2002b) The ATVV intervention produced a change in behavioral states from sleep to alert in clinically stable infants between 33 and 35 weeks post menstrual age (PMA), as well as in very-low-birthweight infants born between 23 and 31 weeks gestational age at birth with and without central nervous system injury (White-Traut et al., 2005; White-Traut et al., 2002b; White-Traut et al., 1993; White-Traut & Pate, 1987). In one study, infants who received the ATVV intervention showed more orally directed behaviors during the intervention and also exhibited more alertness after the intervention when compared to a routine care group (White-Traut et al., 2005). A trend toward decreased feeding times was noted for the ATVV intervention group (White-Traut et al., 2002b). The findings support the use of the ATVV intervention prior to feeding as a means of increasing the frequency of orally directed behaviors, transitioning behavioral states from sleep to alertness, and increasing the proportion of time in an alert behavioral state. In addition to influencing infant behavioral organization, the ATVV intervention yielded a steady decline in cortisol levels in healthy full-term infants when compared to infants who received tactile only intervention or no intervention (White-Traut, Schwertz, McFarlin, & Kogan, 2009). When compared to kangaroo care, the ATVV intervention was successful in transitioning behavioral states from sleep to alertness and increasing the frequency of social interactive behaviors (e.g. engagement and disengagement behaviors), thereby providing an opportunity for social interaction with parents and caregivers for very-low-birthweight infants (White-Traut, Wink, Minehart, & Holditch-Davis, 2012).
While previous research has demonstrated the ability of the ATVV intervention to support alert behavioral states both prior to and after feeding, less is known regarding how the ATVV or other interventions might also influence the incidence of orally directed behaviors, especially when offered prior to oral feeding. Additionally, this is the first research to evaluate infant behavioral responses to the H-HOPE intervention. With the addition of the maternal participatory guidance in conjunction with the ATVV intervention, it was hypothesized that by facilitating the mother’s recognition of her preterm infant’s behavioral cues and support for her decision to change her behavior in accordance with her infant’s cues, infant behavior would be better facilitated (than the ATVV alone) prior to feeding. Thus, the purpose of this study was to determine whether the integrated H-HOPE intervention (that included the ATVV intervention) for preterm infants would increase the frequency of orally directed behaviors and the proportion of time spent in an alert behavioral state when offered prior to oral feeding.
Methods and Procedure
Design
The data presented here are from a randomized clinical trial that employed a balanced two-group randomized trial design (Hinkelmann, 1994; Keppel, 1982; Kirk, 1995) to evaluate preterm infants’ behavioral organization following an integrated H-HOPE intervention. Preterm infants born at 29–34 gestational age (GA) and their mothers, who had at least two social-environmental risk factors, were randomly assigned to the H-HOPE intervention or the Attention Control group (investigators spent a similar amount of time with the mothers in both groups and provided different educational content for mothers than what was provided in the H-HOPE intervention). Orally directed behaviors and behavioral states were assessed at baseline (Day 0, prior to the initiation of the integrated H-HOPE intervention) and weekly prior to feeding during hospitalization (when infants were able to feed orally) until hospital discharge.
Sample and Setting
This research was conducted at two inner city community hospitals, one with a Level II and one with a Level III Special Care Nursery. The standard of care received by both H-HOPE intervention and Attention Control groups at each recruitment site included developmentally supportive nursery care. Infants met the eligibility criteria if they were born between 29 and 34 weeks GA at birth, had no other major health problems, and were clinically stable at enrollment. Infant exclusion criteria included congenital anomalies, necrotizing enterocolitis, brain injury, chronic lung disease, HIV, and prenatal drug exposure. Mothers were eligible if they had at least two social-environmental risk factors which included: self-identified as African-American or Latina, less than high school education, less than 18 years old, history of current mental illness, depression, family income less than 150% of poverty, more than one child under 24 months, four or more children under four in the household, or resided in a disadvantaged neighborhood. A mother was excluded if she had a positive screen for illicit drug use or was not the legal guardian of her infant.
Two hundred-thirty mother-infant dyads were enrolled in the study. Twenty-six were deemed ineligible due to infant health conditions identified after enrollment (Figure 1). Ethnic diversity was limited (Latina = 50.5%; African-American = 45.5%, Table 1), thus data for infants of three women self-identified as being Asian or White were not included in the analysis. In addition, three mothers withdrew after consent and before data collection. After random assignment of the remaining 198 infants, an additional seven infants who developed health conditions in the hospital that were known to interfere with feeding (e.g. pulmonary hypertension, bronchopulmonary dysplasia) or were transferred to another institution were excluded. Five more infants were discharged from the hospital prior to baseline data collection and the start of the intervention and one additional infant was excluded because of a departure from protocol for the baseline observation. The remaining infants in the final analytic sample included 90 intervention and 95 controls.
Figure 1.
Enrollment, Randomization, and Final Sample Size
Table 1.
Baseline Characteristics for Infants Assigned to the H-HOPE Intervention Group and the Attention Control Group
| Infant Characteristics | H-HOPE (n=90) % |
Attention Control (n=95) % |
|
|---|---|---|---|
| Sex | |||
| Female | 57.8 | 45.3 | |
| Male | 42.2 | 54.7 | |
| Plurality | |||
| Singleton | 85.6 | 84.2 | |
| Twin/Triplet | 14.4 | 15.8 | |
| Type of delivery | |||
| NSVD | 54.4 | 52.1 | |
| C-SEC | 45.6 | 47.9 | |
| Site | |||
| A | 55.6 | 57.9 | |
| B | 44.4 | 42.1 | |
| Small for gestational age | |||
| Yes | 30.0 | 29.5 | |
| No | 70.0 | 70.5 | |
| Maternal race/ethnicity | |||
| Latina | 50.0 | 51.6 | |
| African-American | 50.0 | 48.4 | |
|
| |||
| H-HOPE Mean (SD) | Attention Control Mean (SD) | ||
|
| |||
| Gestational age, weeks | 32.6 (1.5) | 32.6 (1.4) | |
| Birthweight, grams | 1800 (330) | 1841 (380) | |
| Apgar score 5 min | 8.3 (1.0) | 8.3 (1.1) | |
| Infant morbidity score at delivery (POPRAS score) | 66.0 (17.7) | 69.8 (19.7) | |
| Chronological age at baseline, days | 8.9 (6.2) | 9.5 (6.7) | |
| Postmenstrual age at baseline, weeks | 33.8 (1.0) | 34.0 (1.0) | |
p > 0.05 (non-significant) for comparison of all infant characteristics by group.
Research participants were African American (n = 91) and Latina (n = 94). There were 90 male infants and 95 female infants. Mean infant gestational age at birth was 32.6 weeks (SD = 1.4). Mean infant birthweight was 1821 grams (SD = 356). The mean chronological age of the infants at baseline was 9.2 days (SD = 6.5). The mean five-minute Apgar score was 8.3 (SD = 1.0). The mean infant health status score from the Problem Oriented Perinatal Risk Assessment System (Davidson & Hobel, 1978; Ross, Hobel, Bragonier, Bear, & Bemis, 1986) (POPRAS) was 68.0 (SD = 18.8). There were no significant differences between the two groups for the infant characteristics. See Table 1 for additional infant characteristics of the sample by groups.
The Integrated H-HOPE Intervention
The H-HOPE intervention integrated the ATVV intervention with the maternal participatory guidance component (Burns et al., 1994). The goal of the maternal participatory guidance component was to facilitate the mother’s recognition of her preterm infant’s behavioral cues and support her decision to change her behavior in accordance with her infant’s cues (White-Traut & Norr, 2009). The overall goal of the ATVV and participatory guidance components was to enhance the infant’s behavioral organization prior to feeding.
The infant directed ATVV component
The infant directed ATTV component of the integrated H-HOPE intervention provided 10 minutes of the following stimuli: auditory (infant directed mother’s voice), tactile (moderate touch stroking or massage) and visual (eye to eye), followed by five minutes of vestibular (horizontal rocking) (Burns et al., 1994). The stimuli are presented in a gradual progression: auditory only for the first 30 seconds, followed by combined auditory and tactile stimuli, with visual added as the infant becomes alert. The vestibular stimuli are added and the tactile component withdrawn for the remaining five minutes. The intervention began when the infant reached 32 weeks PMA or upon entry into the study for infants born at 33–34 weeks. The intervention was administered twice daily prior to feeding by the mother or the research nurse (when the mother was unable to visit the hospital).
The maternal participatory guidance component
The maternal participatory guidance component of the integrated H-HOPE intervention consisted of maternal education and social support through individualized participatory guidance (White-Traut & Norr, 2009). The purpose of adding in the parent participatory guidance component of the intervention was to increase maternal knowledge and understanding of her infant’s behavior and help support the mother to change her behavior in response to her infant’s behaviors so that she could help maintain optimal infant behavioral organization, e.g. not exhibit signs of stress. This component of the intervention was provided by the Nurse/Advocate Team (NAT). The NAT consisted of one nurse and one trained community member and included a bilingual/bicultural Latina and an African-American to provide cultural congruency with the majority of the mothers who participated. The inpatient NAT component was provided during two in-hospital visits. During the first hospital visit, the NAT taught the mother the ATVV, including how to recognize her infant’s behavioral states, orally directed behaviors, engagement and disengagement behavioral cues while administering the ATVV. At both hospital visits, mothers gave a return demonstration of the ATVV to document continued reliability. During the second hospital visit, the NAT reviewed infant behavioral states and orally directed behaviors, taught the mother how to facilitate her baby’s behavior and modify her behavior in response to her baby’s behavioral cues. Mothers learned strategies to soothe their babies when they were showing signs of irritability. Of the women assigned to the H-HOPE intervention group, 63% participated in both of the visits while the infant was hospitalized. For the remaining 37% of mother-infant dyads, infants were discharged from the hospital before the second visit with the NAT team could be completed with the mother.
Research staff and the mothers were taught the ATVV and the maternal participatory guidance components of the H-HOPE intervention to criterion of > 90% agreement. During inpatient administration of H-HOPE, inter-rater reliability with the reliability checklist was maintained above 90% for the mothers and the research staff. Approximately 70% of infants received the H-HOPE twice per day for over half of the days during their hospital stay. Of the remaining infants, 87% were administered the intervention at least once per day, for over half of their days in the hospital.
The Attention Control: Parent Education Program - Infant Safety Education
The Attention Control intervention was designed to provide a similar amount of contact with the mother and staff attention, yet with content distinctly different from the integrated H-HOPE intervention. In addition to receiving the standard of care, mothers of infants assigned to the Attention Control intervention received educational content that included preterm infant care (e.g. bathing, sleep positions, sleep habits, holding the baby), safety of infant equipment and car safety.
Procedure
Baseline data were collected on Day 0. The ATVV intervention was initiated on the following day for infants assigned to the integrated H-HOPE intervention group. Orally directed behaviors and behavioral states were collected using a video recording obtained weekly for 30 minutes prior to starting a late morning feeding. (See Figure 2 for the timeline for data collection and analysis.) The data collection session was conducted in the same manner for both the H-HOPE intervention group (who were simultaneously receiving the ATVV intervention on all days after the baseline assessment) and the Attention Control group. For infants in the H-HOPE intervention group, video recording included five minutes of pre-intervention, 15 minutes during the intervention, and an additional 10 minutes after completion of the intervention during preparation of the infant for the feeding.
Figure 2.

Timeline for Data Collection and Analysis
Prior to the data collection session, the infant was moved from the NICU to an adjacent observation room and placed under a radiant warmer. The infant then remained under the warmer for the first three time segments. The infant was undisturbed for the pre-intervention (first time segment), then during the intervention (second time segment), the ATVV component was administered for infants assigned to the H-HOPE intervention. The infant was left undisturbed for the 10 minutes immediately after the intervention (third time segment). Immediately prior to the feeding, the infant was moved to the evaluation nurse’s arms for the last minute, just prior to the nipple being introduced (fourth time segment). Infants remained on their cardiac monitors during the time segments. All infants had a minimum of two infant behavioral organization assessments (orally directed behaviors and behavioral states), at baseline and before hospital discharge.
Each 30-minute recording was processed through specialized Interact software (Mangold Interact, Germany) that offers the ability to customize coding schemes, time code, and enter the coded data directly into a database that captures all subjects. Due to the complexities and unpredictability of studying preterm infants and the obvious need to prioritize their well-being over the study protocol, the videotaping protocol changed over time to best accommodate the infants, and sometimes periods during the recordings were not code-able because the infant was being blocked by a nurse who was adjusting the equipment or monitors. Also, the video camera needed to be moved at different times to best capture the infant’s face if s/he moved. Therefore, to standardize across infants, we used a representative time period for each segment since we didn’t have a full 5 or 15 minutes of code-able recording across all infants.
Resulting videos were segmented into four time segments: pre-intervention (3 minutes), during intervention (13 minutes), immediately after intervention (1 minute), and immediately prior to feeding (1 minute). Orally directed behaviors and behavioral states were later judged by the Infant Behavior Research Assistant (RA) who was blinded to the purpose of the study.
Measures
Infant behavioral organization
The infant’s behavioral organization was examined using two indicators that included orally directed behaviors and infant behavioral states.
Orally directed behaviors
The frequencies of nine orally directed behaviors (mouthing, tonguing, rooting, sucking on tongue, empty sucking, swipes at mouth, hand to mouth, sucking on hand, and yawning) were measured using the Cagan Videotape Coding System for Orally Directed Behaviors (Cagan, 1995; White-Traut et al., 2005, 2002b). Each time segment was divided into 5-second intervals, for which the count of orally directed behaviors was coded. The total number of orally directed behaviors demonstrated in each 5-second interval was summed across intervals for each of the four time segments (pre-intervention, during intervention, immediately after intervention and immediately prior to feeding) and the total number of cues observed during each time segment was treated as a separate outcome in analysis.
Behavioral state
Seven categories of behavioral state (quiet sleep, active sleep, sleep-wake transition, drowsy, alert, non-alert waking activity, and crying) developed by Thoman and modified by Holditch-Davis (Holditch-Davis & Thoman, 1987; Thoman, 1987, 1990; Thoman et al., 1985; Thoman, Korner, & Kraemer, 1976) were studied. These categories exhibit reliable individual differences and accurately profile the behavioral states of young preterm infants during the neonatal period (Holditch-Davis & Edwards, 1998a, 1998b). Each time segment was coded in 15-second intervals for the dominant (≥ 8 seconds) behavioral state. From these codes, the proportion of time spent in an alert state (quiet and active alert were combined) was calculated for each time segment.
Inter-rater Reliability
To establish inter-rater reliability prior to initiation of the study, the research assistants (blinded to the study’s purpose) were trained to criterion (> 85% agreement) to code the frequency of orally directed behavioral cues and behavioral states. To assure continued inter-rater reliability, 25 of the video recordings not showing the ATVV procedure were scored by the second research assistant.
Inter-rater reliability was determined using the intraclass correlation coefficient (ICC) (Fleiss, 1981). Inter-rater reliability can be interpreted as very good to excellent when the ICC is 0.75 or higher [and moderate to good with ICCs between 0.50 and 0.75]. Twenty-five infants were randomly sampled and a second, independent rater coded orally directed behavioral cues for those infants’ second weekly observations using the same method described above. The total number of cues was summed for each segment. A second random sample of 25 infants was drawn and behavioral state was re-coded for that group by an independent rater for the second weekly observation.
For orally directed behavioral cues, inter-rater reliability for the pre-intervention time segment resulted in an ICC of 0.93 (95% CI = 0.84–0.97), and the ICCs were 0.87 (0.72, 0.95), 0.87 (0.65, 0.95) and 0.88 (0.65, 0.95) during intervention, immediately after intervention, and immediately prior to feeding, respectively. For the proportion of time spent in an alert state during the pre-intervention time segment, inter-rater reliability resulted in an ICC of 0.986 (95% CI = 0.968, 0.994), and the ICCs were 0.998 (0.997, 0.999), 0.994 (0.985, 0.998) and 0.993 (0.983, 0.997) during intervention, immediately after intervention, and immediately prior to feeding, respectively.
Statistical Analysis
Descriptive statistics were calculated overall and by groups (H-HOPE vs. Attention Control) using chi-square tests and t-tests to examine group equivalence at baseline. Two-sided tests were used for all hypothesis testing, controlling for a type I error probability of α = 0.05, with results having a p-value between 0.05 and 0.10 noted as a marginal result.
Due to a significant drop in sample after day 14 post-baseline (reduced due to infant discharge from the hospital), we focused all inferences for the outcomes on Days 0, 7, and 14. Means and standard deviations were calculated for the frequency of orally directed behaviors and the proportion of time spent in an alert state in each time segment by day (Day 0: at baseline, Day 7: 7 days post-baseline, Day 14: 14 days post-baseline) for the H-HOPE intervention group and Attention Control group. To test for group differences in means post-baseline, t-tests were conducted.
For both outcomes which were repeatedly measured over time, mixed-effects models were employed to account for correlations between repeated measurements from the same infant. For orally directed behaviors, mixed-effects Poisson regression models were employed to examine the effect of the intervention on the frequency of orally directed behaviors over time. The mean shape of the outcome trajectories over time were examined using polynomial time effects. Interactions between groups and time trend terms were examined to identify the intervention effect over time.
For behavioral state, mixed-effects linear regression models were employed to examine the proportion of each time segment that the infant spent in an alert state, over time. Polynomial time effects were tested but not significant, so linear trends were modeled using an interaction term between groups and time.
For both outcomes, model selections were performed for both fixed covariates and random effects, and random intercept models were shown to provide good fit.
Results
All baseline sample characteristics were not significantly different between groups (Table 1).
Orally directed behaviors
The number of orally directed behavioral cues observed did not differ by group on Day 0 for any of the time periods – pre-intervention, during intervention, immediately after intervention or immediately prior to feeding (Table 2). In general, higher mean values were seen for the H-HOPE versus the Attention Control group at Day 7, but not at Day 14 when the sample size was significantly reduced due to discharge from the hospital (Table 2). For the H-HOPE group, on Day 7, mean cues increased from pre-intervention of 12.6 to 51.8 during the intervention and subsequently reduced to 4.3 immediately after the intervention and increased to 8.9 immediately prior to feeding. In contrast, infants in the Attention Control group, exhibited a smaller increase from pre-intervention of 7.1 to 33.2 during the intervention time period (the time during which the infants in the H-HOPE group were receiving the intervention) and then reduced to 3.2 immediately after the intervention and increased to 5.3 immediately prior to feeding.
Table 2.
Descriptive Statistics for Orally Directed Behaviors and Behavioral States by Experimental Group and Time Segments for Each Day Post-Baseline
| Variable | Pre-Intervention | During Intervention | Immediately After Intervention | Immediately Prior to Feeding |
|---|---|---|---|---|
|
| ||||
| Frequency of orally directed behaviors | mean (SD) | mean (SD) | mean (SD) | mean (SD) |
| Day 0 | ||||
| H-HOPE (n = 89) | 12.1 (11.4) | 47.5 (48.5) | 3.5 (5.1) | 6.1 (8.2) |
| Attention Control (n = 94) | 11.1 (12.2) | 41.1 (43.0) | 4.1 (5.6) | 7.4 (8.8) |
| Day 7 | ||||
| H-HOPE (n = 41) | 12.6 (18.1)* | 51.8 (56.7)* | 4.3 (5.9) | 8.9 (8.5)** |
| Attention Control (n = 48) | 7.1 (8.3) | 33.2 (42.8) | 3.2 (5.9) | 5.3 (6.7) |
| Day 14 | ||||
| H-HOPE (n = 20) | 11.3 (11.2) | 52.2 (50.0) | 6.8 (12.2) | 6.9 (8.2) |
| Attention Control (n = 27) | 11.8 (8.0) | 49.2 (46.4) | 3.8 (6.0) | 7.4 (8.2) |
|
| ||||
| Proportion of time spent in an alert behavioral state | mean (SD) | mean (SD) | mean (SD) | mean (SD) |
|
| ||||
| Day 0 | ||||
| H-HOPE (n = 89) | 0.28 (0.31) | 0.22 (0.31) | 0.19 (0.34) | 0.31 (0.43) |
| Attention Control (n = 94) | 0.22 (0.30) | 0.16 (0.29) | 0.15 (0.33) | 0.25 (0.37) |
| Day 7 | ||||
| H-HOPE (n = 40) | 0.26 (0.36) | 0.26 (0.31)** | 0.28 (0.13)*** | 0.41 (0.43) |
| Attention Control (n = 48) | 0.21 (0.32) | 0.11 (0.22) | 0.06 (0.22) | 0.31 (0.41) |
| Day 14 | ||||
| H-HOPE (n = 20) | 0.25 (0.34) | 0.28 (0.35) | 0.31 (0.42) | 0.58 (0.48) |
| Control (n = 25) | 0.28 (0.39) | 0.24 (0.33) | 0.22 (0.36) | 0.47 (0.46) |
p < 0.10;
p < 0.05;
p < 0.01 (t test comparing groups within time segment and day post-baseline)
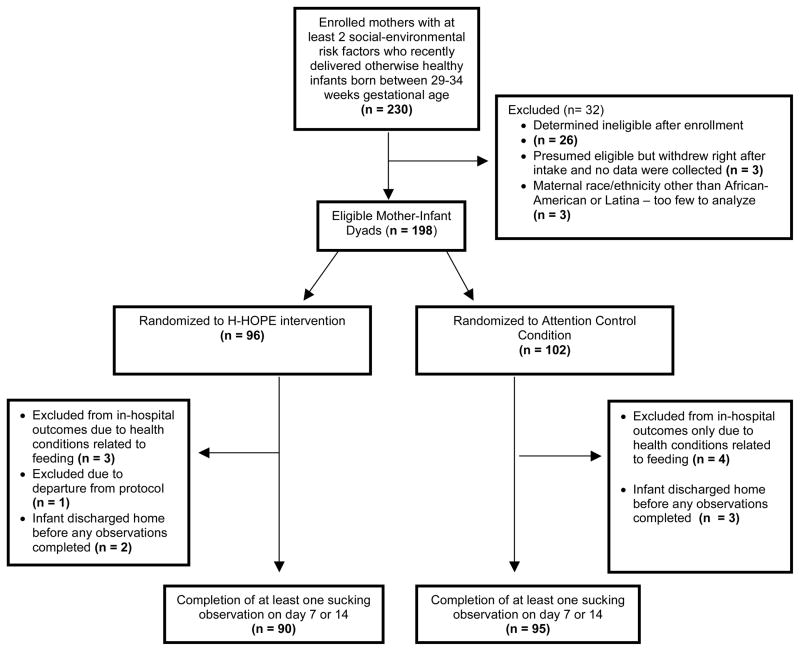
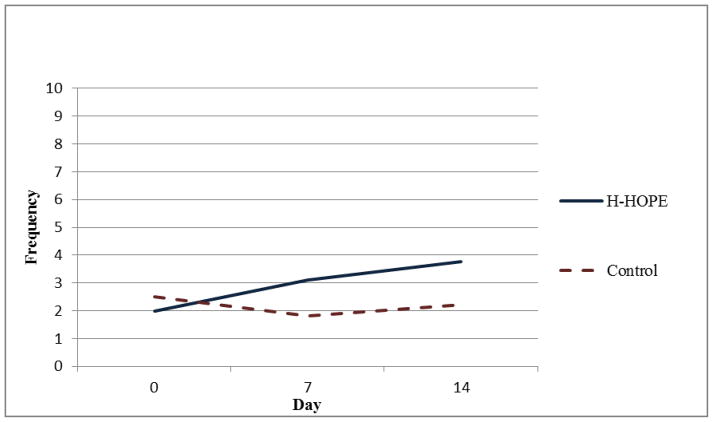
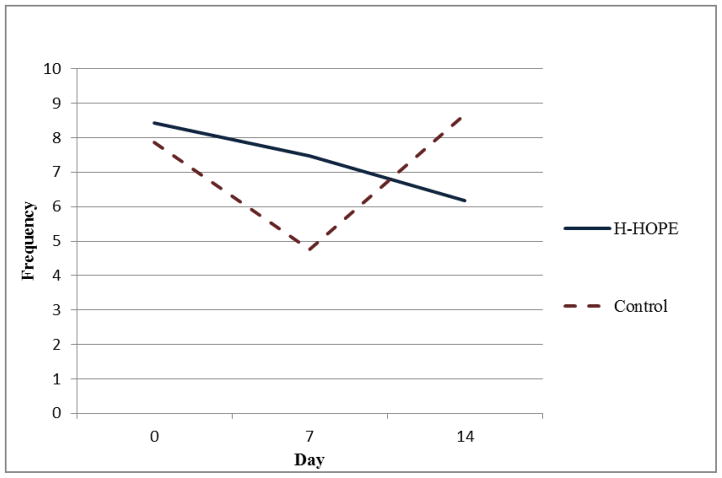
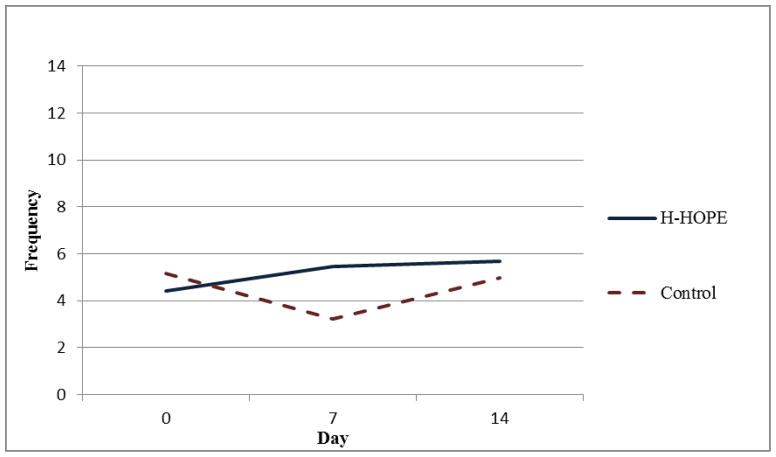
Results of the random-intercept Poisson regression model (Table 3) show that on Day 0 there were no significant group differences in the number of orally directed behavioral cues for any of the four time segments. For all time segments, model results show that the control group exhibited a quadratic pattern over time. That is, in all time segments, the orally directed behavioral cues decreased during earlier days (before Day 7) after randomization, but the decreasing trend leveled off later (after Day 7). Significant group by linear trend and group by quadratic trend terms indicate that the over-time patterns in the intervention group were different from those in the control group. Figures 2 through 5 illustrate the estimated mean of orally directed behavioral cues over time (Day 0–14) for the four time segments. It is evident that the control group’s orally directed behavioral cues showed consistent decreasing patterns between days 0 and 7 before increasing between days 7 and 14. All group by time-trend terms were statistically significant, indicating that the intervention group exhibited different patterns than the control group. Specifically, the intervention group exhibited either a less profound decreasing pattern over time (Figures 3 and 4, pre- and during intervention), or an increasing trend over time (Figures 5 and 6 immediately after intervention and immediately prior to feeding). This finding suggested that the intervention had a stronger effect during the immediately after and the immediately prior to feeding time segments. The same model results showed that a significantly higher number of cues were observed for the H-HOPE compared to control group at Day 7. On Day 14, there is a trend for H-HOPE group trended toward a higher frequency of orally directed behaviors during the immediately after intervention time segments (Table 3; Figures 5).
Table 3.
Longitudinal Model Estimates of Frequency of Orally Directed Behaviors, Beta Estimate (SE)
| Variable | Pre-Intervention | During Intervention | Immediately After Intervention | Immediately Prior to Feeding |
|---|---|---|---|---|
| n infants | 184 | 185 | 157 | 151 |
| n observations | 313 | 318 | 265 | 253 |
|
| ||||
| Intercept | 2.06 (0.11) | 3.24 (0.12) | 0.91 (0.15) | 1.64 (0.12) |
| Group | 0.07 (0.16) | 0.19 (0.17) | −0.22 (0.22) | −0.16 (0.18) |
| day | −0.15 (0.02) *** | −0.11 (0.009)*** | −0.08 (0.03)** | −0.13 (0.02)*** |
| Group* day | 0.01 (0.001) *** | 0.008(0.001)*** | 0.16 (0.05)*** | 0.009 (0.002)*** |
| day* day | 0.14 (0.03) *** | 0.11 (0.01)*** | 0.005 (0.002)** | 0.17 (0.04)*** |
| Group* day* day | −0.01 (0.002)*** | −0.008 (0.0009)*** | −0.008 (0.003)** | −0.01 (0.002)*** |
|
| ||||
| Estimated mean difference between groups at different time points (H-HOPE group - Attention Control group) | ||||
|
| ||||
| Exp(β) (95% CI) | Exp(β) (95% CI) | Exp(β) (95% CI) | Exp(β) (95% CI) | |
|
| ||||
| Day 0 | 1.07 (0.78, 1.47) | 1.21 (0.85, 1.70) | 0.80 (0.52, 1.25) | 0.85 (0.60, 1.22) |
| Day 7 | 1.57 (1.11, 2.21)** | 1.76 (1.24, 2.51)*** | 1.72 (1.04, 2.84)** | 1.69 (1.13, 2.51)** |
| Day 14 | 0.71 (0.49, 1.03)* | 1.20 (0.84, 1.72) | 1.69 (0.97, 2.95)* | 1.14 (0.72, 1.81) |
Note. N =184, total 312 observations. All models were random intercept-only models
p < 0.10;
p < 0.05;
p < 0.01
Figure 5.
Mean Frequency of Orally Directed Behaviors Immediately After Intervention
Figure 3.
Mean Frequency of Total Orally Directed Behaviors Pre-Intervention
Figure 4.
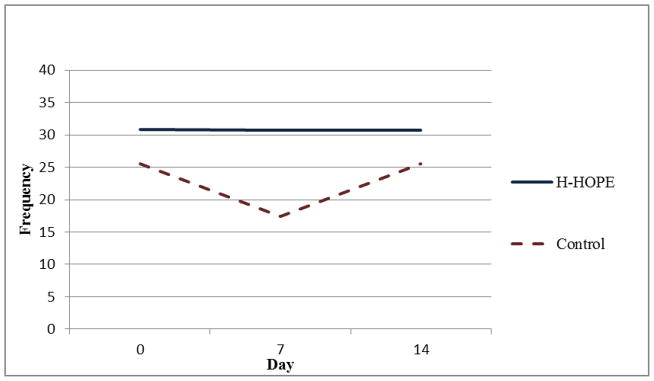
Mean Frequency of Orally Directed Behaviors During Intervention
Figure 6.
Mean Frequency of Orally Directed Behaviors Immediately Prior to Feeding
Infant behavioral state
The proportion of time in an alert state also did not differ by group at baseline (Day 0, before the intervention began) (Table 2). Higher mean values were noted for the H-HOPE versus the Attention Control groups at Day 7, with significant differences identified during and after the intervention. While the proportion of time spent alert was higher after intervention and prior to feeding, there were no significant group differences at Day 14 (Table 2).
On Day 7, the mean proportion of time spent in an alert state for the H-HOPE infants remained stable at pre-intervention and during intervention (0.26), increased to 0.28 immediately after intervention, and further increased to 0.41 immediately prior to feeding. In contrast, infants in the Attention Control group, exhibited a decrease in alertness from pre-intervention of 0.21 to 0.11 during the intervention time period (the time that the infants in the H-HOPE group were receiving the intervention) and then reduced to 0.06 immediately after the intervention and increased to 0.31 immediately prior to feeding.
Results of the random-intercept linear regression model (Table 4) show that there were no group differences on Day 0 for the four time segments in the proportion of time in an alert state. However, the proportion of time in alert state for all four time segments had linear growth over time. Although the intervention group was estimated to have a faster increase than the control group for all time segments, except for pre-intervention, such differences were not statistically significant (p-value for group by day > 0.05). However, significantly higher mean proportions of time spent in an alert state were observed for the H-HOPE compared to control group at Day 7 both during and immediately after the intervention. On Day 14, there was a trend for an increase in the proportion of time spent in an alert state for the H-HOPE group when compared with the Attention Control group for the immediate after intervention time segment (Table 4; Figure 7).
Table 4.
Longitudinal Model Estimates for Proportion of Time Spent in an Alert Behavioral State, Beta Estimate (SE)
| Variable | Pre-Intervention | During Intervention | Immediately After Intervention | Immediately Prior to Feeding |
|---|---|---|---|---|
| n infants | 184 | 185 | 157 | 150 |
| n observations | 312 | 317 | 264 | 251 |
|
| ||||
| Intercept | 0.21 (0.03) | 0.15 (0.03) | 0.13 (0.04) | 0.25 (0.05) |
| Group | 0.06 (0.05) | 0.07 (0.04) | 0.06 (0.05) | 0.06 (0.07) |
| day | 0.003 (0.005) | 0.003 (0.004) | 0.001 (0.005) | 0.01 (0.006)** |
| Group* day | −0.005 (0.007) | 0.001 (0.007) | 0.008 (0.008) | 0.005 (0.01) |
|
| ||||
| Estimated mean difference between groups at different time points (H-HOPE group - Attention Control group) | ||||
|
| ||||
| Day 0 | 0.06 (0.05) | 0.07 (0.04) | 0.06 (0.05) | 0.06 (0.06) |
| Day 7 | 0.03 (0.04) | 0.08 (0.04)** | 0.12 (0.05)** | 0.10 (0.07) |
| Day 14 | −0.008 (0.08) | 0.09 (0.07) | 0.18 (0.09)* | 0.13 (0.12) |
Note. N =184, total 312 observations. All models were random intercept-only models
p < 0.10;
p < 0.05;
p < 0.01
Figure 7.
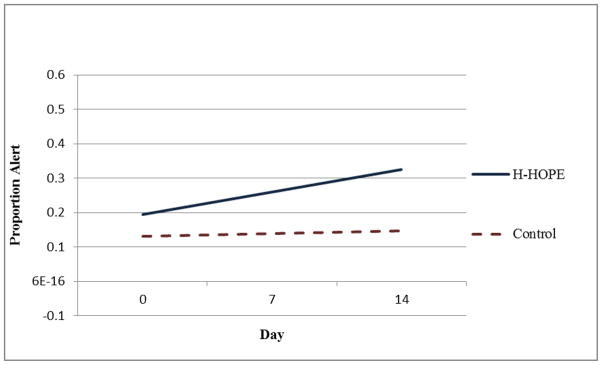
Mean Proportion of Time Spent in an Alert Behavioral State Immediately After Intervention
Infant characteristics on Day 14
Overall, findings for Day 14 may have been influenced by infant characteristics. Infants remaining in the hospital on Day 14 post-baseline were more likely than those who had already been discharged to be twins (20.5 vs. 6.3%, respectively, p = 0.047), SGA (38.5% vs. 20.8%, p = 0.07), Latina (56.4 vs. 37.5%, p = 0.08), have lower mean GA at birth (31.9 vs. 32.6%, p = 0.02), have lower mean birthweight (1636 vs. 1842, p = 0.003), and have higher mean POPRAS scores (78.6 vs. 63.6, p = 0.0002). They did not differ significantly with respect to other infant characteristics, such as sex, site, and five-minute Apgar score.
Discussion
The current study is the first to identify a pattern of increased frequency of orally directed behaviors in the infants who were assigned to the H-HOPE intervention group when compared to infants assigned to the Attention Control group. Particularly, on Day 7, the integrated H-HOPE intervention facilitated an increase in the frequency of orally directed behaviors throughout the pre-intervention, during intervention, and immediately prior to feeding time segments. As expected, the integrated H-HOPE intervention was able to facilitate the transition to the alert state at Day 7 for both groups during the intervention and the immediately after intervention time segments. At Day 14, infants assigned to the integrated H-HOPE intervention trended toward an increase frequency of orally directed behaviors and proportion of time spent in alert behavioral state for immediately after intervention. Day 14 findings are likely related to infant characteristics for those infants remaining in the hospital. Improved behavioral organization immediately prior to feeding has the potential to facilitate complete oral feeding (Erlandsson et al., 2007; Hentschel et al., 2007; Matthiesen et al., 2001; Ransjo-Arvidson et al., 2001; Widstrom et al., 1987). However, there are there are no other feeding readiness interventions that address changes in orally directed behaviors and behavioral state. One study recently compared the frequency of alert behavioral states after infants received the ATVV intervention or Kangaroo Care (Holditch-Davis et al., Submitted). The ATVV intervention group infants had significantly more alert states when compared to infants assigned to Kangaroo care. The data from the current study are suggestive that the H-HOPE intervention facilitates infant behavioral organization prior to feeding and offers support for the use of the H-HOPE intervention prior to feeding as a means of increasing the frequency of orally directed behaviors as well as alert behavioral states.
While the infant directed component of the H-HOPE intervention, the ATVV, is known to facilitate the transition of behavioral states from sleep to alertness in clinically stable premature infants and those with central nervous system injury (White-Traut et al., 2005; White-Traut et al., 2002a; White-Traut et al., 2002b; White-Traut, Nelson, Silvestri, Cunningham, & Patel, 1997; White-Traut & Pate, 1987) less is known regarding the incidence of orally directed behaviors following the ATVV intervention that is offered prior to feeding (White-Traut et al., 2005; White-Traut et al., 2002b) or whether this intervention leads to improved oral feeding. Prior research with full term infants has indicated that orally directed behavioral cues are critical indicators of organized oromotor neurobehaviors, and thus may play a significant role in the infant’s ability to communicate readiness to feed as well as sustain successful breast feeding (Bell et al., 2012; Brazelton & Nugent, 1995; Cagan, 1995; White-Traut et al., 2005). While White-Traut et al. (2005) demonstrated that orally directed behavioral cues prior to feeding is correlated with oral feeding efficiency in premature infants, there is limited research regarding the relationship of orally directed behaviors with the infant’s ability to feed orally in both premature infants and full-term infants. The findings reported here provide initial support for the use of H-HOPE intervention prior to feeding as a means of increasing frequency of orally directed behaviors. Further research is warranted to understand any additional benefits of these findings on oral feeding efficiency.
Research has shown that the alert behavioral state is correlated with successful oral feeding (the ability to complete the entire feeding mouth) (Medoff-Cooper, Verklan, & Carlson, 1993; Panniers, 2002; Pickler, Mauck, & Geldmaker, 1997; White-Traut et al., 2002a; White-Traut et al., 2002b). Largely, the alert behavioral state has direct effects on the rate of sucks and harder nutritive sucking pressure, higher quantitative non-nutritive sucking with a more complex pattern of burst organization as well as greater consistency of sucking patterns (Bingham, Ashikaga, & Abbasi, 2011; McGrath & Medoff-Cooper, 2002; Medoff-Cooper & Ray, 1995; Panniers, 2002). In the current study, infants in the H-HOPE group exhibited a higher proportion of an alert state only during intervention and immediately after intervention on Day 7. This finding was expected and is suggestive that H-HOPE may be a benefit in facilitating the transition from sleep to alertness around the time of feeding. Alert infant behavior also provides mothers with the opportunity to interact with their infants.
Cue-based feeding protocols often include assessment of orally directed behavioral cues. Prior research is suggestive that cue-based protocols improve oral feeding intake, shorter oral feeding progression, and shorter hospitalization (Jadcherla et al., 2012; Kirk, Alder, & King, 2007; Puckett, Grover, Holt, & Sankaran, 2008; Saunders, Baker-Friedman, & Stramoski, 1990; Shaker, 2012). Behavioral cues that are often used for assessment of infant readiness to feed include but are not limited to rooting, hand to mouth, sucking on hand, alert states, and inability to settle after position change or diaper change (Jadcherla et al., 2012; Kirk et al., 2007; Puckett et al., 2008; Shaker, 2012). The ATVV and the new integrated H-HOPE intervention have demonstrated support of infant behavioral organization prior to feeding and with future research has the potential, in conjunction with cue-based feeding protocols, to improve oral feeding outcomes.
Thoyre has recently developed a micro-chip that amplifies the infant’s oral and esophageal sounds. As in the H-HOPE intervention, mothers learn to read and respond to the infant’s feeding behavior and sounds through participatory guidance. When the mother correctly reads and responds to these feeding sounds, infant behavioral organization during feeding is improved (Thoyre, Park, Pados, & Hubbard, 2013; Thoyre, Holditch-Davis, Schwartz, Melendez Roman, & Nix, 2012). Data from the H-HOPE intervention and Thoyre’s research support the implementation of participatory guidance with mothers as crucial for learning infant behavioral organization surrounding feeding. In Thoyre’s research, participatory guidance serves to support successful oral feeding. Future research with the H-HOPE intervention is warranted to better understand if this approach facilitates successful oral feeding.
The behavioral state findings are slightly different than previously reported. For this particular study, the protocol was changed from our prior protocol. Both groups of infants were moved from the NICU to the observation room, thus this may account for the beginning of state transition regardless of receipt of intervention. Also, the mean counts of orally directed behaviors were only marginally different between the groups at Day 14 in the after intervention time segment, and not significantly different at any of the other time segments on this particular day. By Day 14, many of the infants were discharged to home while the infants remaining in the hospital were more likely to be twins, younger at birth, SGA, have lower birthweights, and higher POPRAS scores that required continued hospitalization. Therefore, the lack of significant findings on Day 14 may be related to the lower number of infants available for analyses (approximately two times lower), health status, and related characteristics.
Study limitations include the sample size, the narrow age range for the infants, and transporting infants out of the NICU to the observation room prior to the data collection session. This was the first protocol where we moved infants out of the NICU to an observation room for the data collection session. Transferring infants from the NICU most likely influenced the infant’s behavioral state prior to the data collection session with more infants alert at baseline regardless of group assignment. The number of infants remaining in the hospital at 14 days was low (two times lower than Day 7) which diminished the capacity for our analyses. Additionally, these infants were likely to be less healthy, requiring continued hospital care and reducing their behavioral capacity. Finally, infants in the study had a narrow range of post menstrual age during their participation on the study which limits the generalizability of our findings to infants of younger and older ages. A major strength of the study is the use of two different measures of infant behavioral organization as a means to assess feeding readiness. Orally directed behavioral cues may be more valid than behavioral states for assessment of feeding readiness.
Future Research
Our findings are suggestive that the integrated H-HOPE intervention facilitated infant behavioral organization for clinically stable infants born between 29 and 34 weeks GA. However, future research with the integrated H-HOPE intervention that measures additional behavioral indicators for feeding readiness and feeding outcomes is warranted. Little prior research has measured orally directed behaviors prior to feeding. Based on the current findings, additional research is needed to better understand the role of these behavioral cues in relationship to readiness for oral feeding and for progression towards successful oral feeding and feeding efficiency. Additionally, the study of control versus prefeeding interventions will advance our understanding of the utility of such interventions. The findings presented here support the use of maternal participatory guidance, particularly in regard to supporting optimal infant behavioral organization. Future research is critical to identify interventions that improve oral feeding skills, especially for more fragile preterm and full-term infants who are at greater risk for organizing their behaviors prior to feeding or who have difficulty with oral feeding progression.
Implications
Intervention that builds the preterm infant’s capacity for successful oral feeding is critically needed (Lau & Smith, 2012; Pickler et al., 2010; Pickler, Reyna, Griffin, Lewis, & Thompson, 2012; Thoyre et al., 2012). The data reported in the current study are suggestive that offering a pre-feeding intervention may enhance the infant’s preparation for oral feeding by increasing the frequency of orally directed behaviors and proportion of time spent in an alert behavioral state. The ATVV intervention has previously been shown to be relatively easy for parents and clinicians to learn (White-Traut & Nelson, 1988) and is developmentally appropriate (Burns et al., 1994). An added value of the integrated H-HOPE intervention in addition to ATVV component is the implementation of maternal participatory guidance to support infant behavioral organization (White-Traut & Norr, 2009; White-Traut, Nelson, Burns, & Cunningham, 1994). Thus, the integrated H-HOPE intervention has the potential for supporting infant behavioral organization prior to feeding and has the additional benefit of providing a guided opportunity for mothers to engage with their infants surrounding feeding. Orally directed behaviors appear to be an important indicator of infant preparation for feeding. When orally directed behavioral cues are used in conjunction with assessment of behavioral states, both can be especially valuable assessment tools to the clinicians. Use of this combined approach to practice would strengthen clinician assessment for initiation of (beginning the first oral feeding) and daily preparation for oral feeding in preterm infants and highly recommended.
Acknowledgments
This work was supported by grants from the National Institutes of Child Health and Human Development, the National Institute of Nursing Research (1 R01 HD050738-01A2), and the Harris Foundation to the University of Illinois at Chicago. We also wish to acknowledge the infants who participated in this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ardura J, Andres J, Aldana J, Revilla MA. Development of sleep-wakefulness rhythm in premature babies. Acta Pædiatrica. 1995;84(5):484–489. doi: 10.1111/j.1651-2227.1995.tb13679.x. [DOI] [PubMed] [Google Scholar]
- Bell AF, Lucas R, White-Traut RC. Concept clarification of neonatal neurobehavioural organization. Journal of Advanced Nursing. 2008;61(5):570–581. doi: 10.1111/j.1365-2648.2007.04561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AF, White-Traut R, Rankin K. Fetal exposure to synthetic oxytocin and the relationship with prefeeding cues within one hour postbirth. Early Human Development. 2012;89(3):137–143. doi: 10.1016/j.earlhumdev.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Bingham PM, Ashikaga T, Abbasi S. Relationship of behavioral state and tube-feeding to non-nutritive sucking in premature infants. Journal of Neonatal Nursing. 2011;17:150–157. [Google Scholar]
- Brazelton TB, Nugent JK. Neonatal Behavioral Assessment Scale. Vol. 3. London: Mac Keith Press; 1995. [Google Scholar]
- Burns K, Cunningham N, White-Traut R, Silvestri J, Nelson MN. Infant stimulation: modification of an intervention based on physiologic and behavioral cues. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 1994;23(7):581–589. doi: 10.1111/j.1552-6909.1994.tb01924.x. [DOI] [PubMed] [Google Scholar]
- Cagan J. Feeding readiness behavior in preterm infants. Neonatal Network. 1995;14(2):82. [Google Scholar]
- Davidson EC, Hobel CJ. POPRAS: A guide to using the prenatal, intrapartum, postpartum record. Torrence, CA: South Bay Regional Perinatal Project Professional Staff Association; 1978. [Google Scholar]
- Duffy FH, Als H, McAnulty GB. Behavioral and electrophysiological evidence for gestational age effects in healthy preterm and fullterm infants studied two weeks after expected due date. Child Development. 1990;61(4):271–286. [PubMed] [Google Scholar]
- Erlandsson K, Dsilna A, Fagerberg I, Christensson K. Skin-to-skin care with the father after cesarean birth and its effect on newborn crying and prefeeding behavior. Birth. 2007;34(2):105–114. doi: 10.1111/j.1523-536X.2007.00162.x. [DOI] [PubMed] [Google Scholar]
- Fleiss JL. Statistical methods for rates and proportions. 2. New York: John Wiley; 1981. [Google Scholar]
- Foreman SW, Thomas KA, Blackburn ST. Individual and gender differences matter in preterm infant state development. Journal Of Obstetric, Gynecologic & Neonatal Nursing. 2008;37(6):657–665. doi: 10.1111/j.1552-6909.2008.00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy T, Czyzewski D, Phillips S, Ligon B, Baldwin J, Klish W. Treatment of severe feeding refusal in infants and toddlers. Infants & Young Children. 1997;9(3):26–35. [Google Scholar]
- Geertsma MA, Hyams JS, Pelletier JM, Reiter S. Feeding resistance after parenteral hyperalimentation. American Journal of Diseases of Children. 1985;139(3):255–256. doi: 10.1001/archpedi.1985.02140050049020. [DOI] [PubMed] [Google Scholar]
- Gill NE, Behnke M, Conlon M, Anderson GC. Nonnutritive sucking modulates behavioral state for preterm infants before feeding. Scandinavian Journal of Caring Sciences. 1992;6(1):3–7. doi: 10.1111/j.1471-6712.1992.tb00115.x. [DOI] [PubMed] [Google Scholar]
- Hentschel J, Ruff R, Juette F, von Gontard A, Gortner L. Neonatal facial movements in the first minutes of life--eye opening and tongue thrust: an observational study. American Journal of Perinatology. 2007;24(10):611–618. doi: 10.1055/s-2007-992178. [DOI] [PubMed] [Google Scholar]
- Hinkelmann KKO. Design and analysis of experiments. New York, NY: J. Wiley; 1994. [Google Scholar]
- Holditch-Davis D. The development of sleeping and walking states in high-risk preterm infants. Infant Behavior and Development. 1990;13:513–531. [Google Scholar]
- Holditch-Davis D, Edwards LJ. Modeling development of sleep-wake behaviors. II. Results of two cohorts of preterms. Physiology and Behavior. 1998a;63(3):319–328. doi: 10.1016/s0031-9384(97)00396-x. [DOI] [PubMed] [Google Scholar]
- Holditch-Davis D, Edwards LJ. Temporal organization of sleep-wake states in preterm infants. Developmental Psychobiology. 1998b;33(3):257–269. doi: 10.1002/(sici)1098-2302(199811)33:3<257::aid-dev6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Holditch-Davis D, Edwards LJ, Helms RW. Modeling development of sleep-wake behaviors: I. Using the mixed general linear model. Physiology and Behavior. 1998;63(3):311–318. doi: 10.1016/s0031-9384(97)00459-9. [DOI] [PubMed] [Google Scholar]
- Holditch-Davis D, Scher M, Schwartz T, Hudson-Barr D. Sleeping and waking state development in preterm infants. Early Human Development. 2004;80(1):43–64. doi: 10.1016/j.earlhumdev.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Holditch-Davis D, Thoman EB. Behavioral states of premature infants: implications for neural and behavioral development. Developmental Psychobiology. 1987;20(1):25–38. doi: 10.1002/dev.420200107. [DOI] [PubMed] [Google Scholar]
- Holditch-Davis D, White-Traut R, Levy JA, O’Shea TM, Geraldo V, David RJ. Maternally administered interventions for preterm infants in the NICU: Effects on maternal psychological distress and mother-infant relationship. doi: 10.1016/j.infbeh.2014.08.005. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingersoll EW, Thoman EB. Sleep/Wake states of preterm infants: Stability, developmental change, diurnal variation, and relation with caregiving activity. Child Development. 1999;70(1):1–10. doi: 10.1111/1467-8624.00001. [DOI] [PubMed] [Google Scholar]
- Jadcherla SR, Peng J, Moore R, Saavedra J, Shepherd E, Fernandez S, DiLorenzo C. Impact of personalized feeding program in 100 NICU infants: pathophysiology-based approach for better outcomes. Journal of Pediatric Gastroenterology and Nutrition. 2012;54(1):62–70. doi: 10.1097/MPG.0b013e3182288766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel G. Design and analysis: A researcher’s handbook. 2. Englewood Cliffs, NJ: Prentice-Hall; 1982. [Google Scholar]
- Kinneer MD, Beachy P. Nipple feeding premature infants in the neonatal intensive-care unit: factors and decisions. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 1994;23(2):105–112. doi: 10.1111/j.1552-6909.1994.tb01859.x. [DOI] [PubMed] [Google Scholar]
- Kirk AT, Alder SC, King JD. Cue-based oral feeding clinical pathway results in earlier attainment of full oral feeding in premature infants. Journal of Perinatology. 2007;27(9):572–578. doi: 10.1038/sj.jp.7211791. [DOI] [PubMed] [Google Scholar]
- Kirk RE. Experimental design: Procedures for the behavioral sciences. Pacific Grove, CA: Brooks/Cole; 1995. [Google Scholar]
- Korner AF. State as variable, as obstacle, and as mediator of stimulation in infant research. Merrill-Palmer Quarterly. 1972;18:77–94. [Google Scholar]
- Lau C. Fundamental of oral feeding skills 2012 [Google Scholar]
- Lau C, Smith EO. Interventions to improve the oral feeding performance of preterm infants. Acta Pædiatrica. 2012;101(7):e269–274. doi: 10.1111/j.1651-2227.2012.02662.x. [DOI] [PubMed] [Google Scholar]
- Lemons PK, Lemons JA. Transition to breast/bottle feedings: the premature infant. Journal of the American College of Nutrition. 1996;15(2):126–135. doi: 10.1080/07315724.1996.10718577. [DOI] [PubMed] [Google Scholar]
- Matthiesen AS, Ransjo-Arvidson AB, Nissen E, Uvnas-Moberg K. Postpartum maternal oxytocin release by newborns: effects of infant hand massage and sucking. Birth. 2001;28(1):13–19. doi: 10.1046/j.1523-536x.2001.00013.x. [DOI] [PubMed] [Google Scholar]
- McGrath JM, Medoff-Cooper B. Alertness and feeding competence in extremely early born preterm infants. Newborn and Infant Nursing Reviews. 2002;2(3):174–186. [Google Scholar]
- Medoff-Cooper B, Bilker W, Kaplan JM. Sucking patterns and behavioral state in 1- and 2-day-old full-term infants. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 2010;39(5):519–524. doi: 10.1111/j.1552-6909.2010.01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff-Cooper B, Ray W. Neonatal sucking behaviors. Image - The Journal of Nursing Scholarship. 1995;27(3):195–200. doi: 10.1111/j.1547-5069.1995.tb00858.x. [DOI] [PubMed] [Google Scholar]
- Medoff-Cooper B, Verklan T, Carlson S. The development of sucking patterns and physiologic correlates in very-low-birth-weight infants. Nursing Research. 1993;42(2):100–105. [PubMed] [Google Scholar]
- Mouradian L, Als H, Coster W. Neurobehavioral functioning of healthy preterm infants of varying gestational ages. Developmental and Behavioral Pediatrics. 2000;21(6):408–416. doi: 10.1097/00004703-200012000-00002. [DOI] [PubMed] [Google Scholar]
- Panniers TL. Refining clinical terminology for expert system development: an application in the neonatal intensive care unit. Pediatric Nursing. 2002;28(5):519–513. 529. [PubMed] [Google Scholar]
- Pickler RH, Best AM, Reyna BA, Gutcher G, Wetzel PA. Predictors of nutritive sucking in preterm infants. Journal of Perinatology. 2006;26(11):693–699. doi: 10.1038/sj.jp.7211590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickler RH, Best AM, Reyna BA, Wetzel PA, Gutcher GR. Prediction of Feeding Performance in Preterm Infants. Newborn and Infant Nursing Reviews. 2005;5(3):116–123. doi: 10.1053/j.nainr.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickler RH, Mauck AG, Geldmaker B. Bottle-feeding histories of preterm infants. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 1997;26(4):414–420. doi: 10.1111/j.1552-6909.1997.tb02723.x. [DOI] [PubMed] [Google Scholar]
- Pickler RH, McGrath JM, Reyna BA, McCain N, Lewis M, Cone S, Best A. A Model of Neurodevelopmental Risk and Protection for Preterm Infants. Journal of Perinatal & Neonatal Nursing. 2010;24(4):356–365. doi: 10.1097/JPN.0b013e3181fb1e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickler RH, Reyna BA, Griffin JB, Lewis M, Thompson AM. Changes in Oral Feeding in Preterm Infants Two Weeks After Hospital Discharge. Newborn and Infant Nursing Reviews. 2012;12(4):202–206. doi: 10.1053/j.nainr.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puckett B, Grover VK, Holt T, Sankaran K. Cue-based feeding for preterm infants: a prospective trial. American Journal of Perinatology. 2008;25(10):623–628. doi: 10.1055/s-0028-1090583. [DOI] [PubMed] [Google Scholar]
- Ransjo-Arvidson AB, Matthiesen AS, Lilja G, Nissen E, Widstrom AM, Uvnas-Moberg K. Maternal analgesia during labor disturbs newborn behavior: effects on breastfeeding, temperature, and crying. Birth. 2001;28(1):5–12. doi: 10.1046/j.1523-536x.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- Riordan MM, Iwata BA, Finney JW, Wohl MK, Stanley AE. Behavioral assessment and treatment of chronic food refusal in handicapped children. Journal of Applied Behavior Analysis. 1984;17(3):327–341. doi: 10.1901/jaba.1984.17-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross MG, Hobel CJ, Bragonier JR, Bear MB, Bemis RL. A simplified risk-scoring system for prematurity. American Journal of Perinatology. 1986;3(4):339–144. doi: 10.1055/s-2007-999893. [DOI] [PubMed] [Google Scholar]
- Saunders RB, Baker-Friedman C, Stramoski PR. Feeding preterm infants: Schedule or demand? Journal of Obstetric, Gynecologic, and Neonatal Nursing. 1990;20(3):212–218. doi: 10.1111/j.1552-6909.1991.tb02533.x. [DOI] [PubMed] [Google Scholar]
- Shaker C. Feeding me only when I’m cueing: Moving away from a volume-driven culture in the NICU. Journal of Perinatology-Neonatology. 2012;25(3):27–32. [Google Scholar]
- Thoman EB. The sleep and waking states of infants: Correlations across time and person. Physiology & Behavior. 1987;41:531–537. doi: 10.1016/0031-9384(87)90307-6. [DOI] [PubMed] [Google Scholar]
- Thoman EB. Sleeping and waking states in infants: A functional perspective. Neuroscience and Biobehavioral Reviews. 1990;14:93–107. doi: 10.1016/s0149-7634(05)80165-4. [DOI] [PubMed] [Google Scholar]
- Thoman EB, Davis DH, Raye JR, Philipps AF, Rowe JC, Denenberg VH. Theophylline affects sleep-wake state development in premature infants. Neuropediatrics. 1985;16:13–18. doi: 10.1055/s-2008-1052537. [DOI] [PubMed] [Google Scholar]
- Thoman EB, Korner AF, Kraemer HC. Individual consistency in behavioral states in neonates. Developmental Psychobiology. 1976;9(3):271–283. doi: 10.1002/dev.420090311. [DOI] [PubMed] [Google Scholar]
- Thoyre S, Park J, Pados B, Hubbard C. Developing a co-regulated, cue-based feeding practice: The critical role of assessment and reflection. Journal of Neonatal Nursing. 2013;19(4):139–148. doi: 10.1016/j.jnn.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoyre SM, Holditch-Davis D, Schwartz TA, Melendez Roman CR, Nix W. Coregulated approach to feeding preterm infants with lung disease: effects during feeding. Nursing Research. 2012;61(4):242–251. doi: 10.1097/NNR.0b013e31824b02ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White-Traut R, Berbaum ML, Lessen B, McFarlin B, Cardenas L. Feeding readiness in preterm infants: the relationship between preterm behavioral states and feeding readiness behaviors and efficiency during transition from gavage to oral feeding. The American Journal of Maternal/Child Nursing. 2005;30(1):52–59. [PubMed] [Google Scholar]
- White-Traut R, Nelson M, Silvestri J, Vasan U, Littau S, Meleedy-Rey P, Patel M. The effect of auditory, tactile, visual, and vestibular intervention on length of stay, alertness, and feeding progression in preterm infants. Developmental Medicine and Child Neurology. 2002a;44(2):91–97. doi: 10.1017/s0012162201001736. [DOI] [PubMed] [Google Scholar]
- White-Traut R, Nelson MN, Silvestri JM, Vasan U, Patel M, Cardenas L. Feeding readiness behaviors and feeding efficiency in response to ATVV intervention. Newborn and Infant Nursing Reviews. 2002b;2(3):166–173. [Google Scholar]
- White-Traut R, Norr K. An ecological model for premature infant feeding. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 2009;38(4):478–489. doi: 10.1111/j.1552-6909.2009.01046.x. quiz 489–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White-Traut R, Wink T, Minehart T, Holditch-Davis D. Frequency of Premature Infant Engagement and Disengagement Behaviors During Two Maternally Administered Interventions. Newborn and Infant Nursing Reviews. 2012;12(3):124–131. doi: 10.1053/j.nainr.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White-Traut RC, Nelson MN. Maternally administered tactile, auditory, visual, and vestibular stimulation: relationship to later interactions between mothers and premature infants. Research in Nursing & Health. 1988;11(1):31–39. doi: 10.1002/nur.4770110106. [DOI] [PubMed] [Google Scholar]
- White-Traut RC, Nelson MN, Burns K, Cunningham N. Environmental influences on the developing premature infant: theoretical issues and applications to practice. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 1994;23(5):393–401. doi: 10.1111/j.1552-6909.1994.tb01896.x. [DOI] [PubMed] [Google Scholar]
- White-Traut RC, Nelson MN, Silvestri JM, Cunningham N, Patel M. Responses of preterm infants to unimodal and multimodal sensory intervention. Pediatric Nursing. 1997;23(2):169–175. 193. [PubMed] [Google Scholar]
- White-Traut RC, Nelson MN, Silvestri JM, Patel MK, Kilgallon D. Patterns of physiologic and behavioral response of intermediate care preterm infants to intervention. Pediatric Nursing. 1993;19(6):625–629. [PubMed] [Google Scholar]
- White-Traut RC, Pate CM. Modulating infant state in premature infants. Journal of Pediatric Nursing. 1987;2(2):96–101. [PubMed] [Google Scholar]
- White-Traut RC, Schwertz D, McFarlin B, Kogan J. Salivary cortisol and behavioral state responses of healthy newborn infants to tactile-only and multisensory interventions. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 2009;38(1):22–34. doi: 10.1111/j.1552-6909.2008.00307.x. [DOI] [PubMed] [Google Scholar]
- Widstrom AM, Lilja G, Aaltomaa-Michalias P, Dahllof A, Lintula M, Nissen E. Newborn behaviour to locate the breast when skin-to-skin: a possible method for enabling early self-regulation. Acta Pædiatrica. 2011;100(1):79–85. doi: 10.1111/j.1651-2227.2010.01983.x. [DOI] [PubMed] [Google Scholar]
- Widstrom AM, Ransjo-Arvidson AB, Christensson K, Matthiesen AS, Winberg J, Uvnas-Moberg K. Gastric suction in healthy newborn infants. Effects on circulation and developing feeding behaviour. Acta Paediatrica Scandinavica. 1987;76(4):566–572. doi: 10.1111/j.1651-2227.1987.tb10522.x. [DOI] [PubMed] [Google Scholar]
- Wolff PH. The development of attention in young infants. Annals of the New York Academy of Science. 1965;118:815–830. doi: 10.1111/j.1749-6632.1965.tb40153.x. [DOI] [PubMed] [Google Scholar]
- Wolff PH. Observations of early development of smiling. Vol. 2. New York: John Wiley; 1966. [Google Scholar]