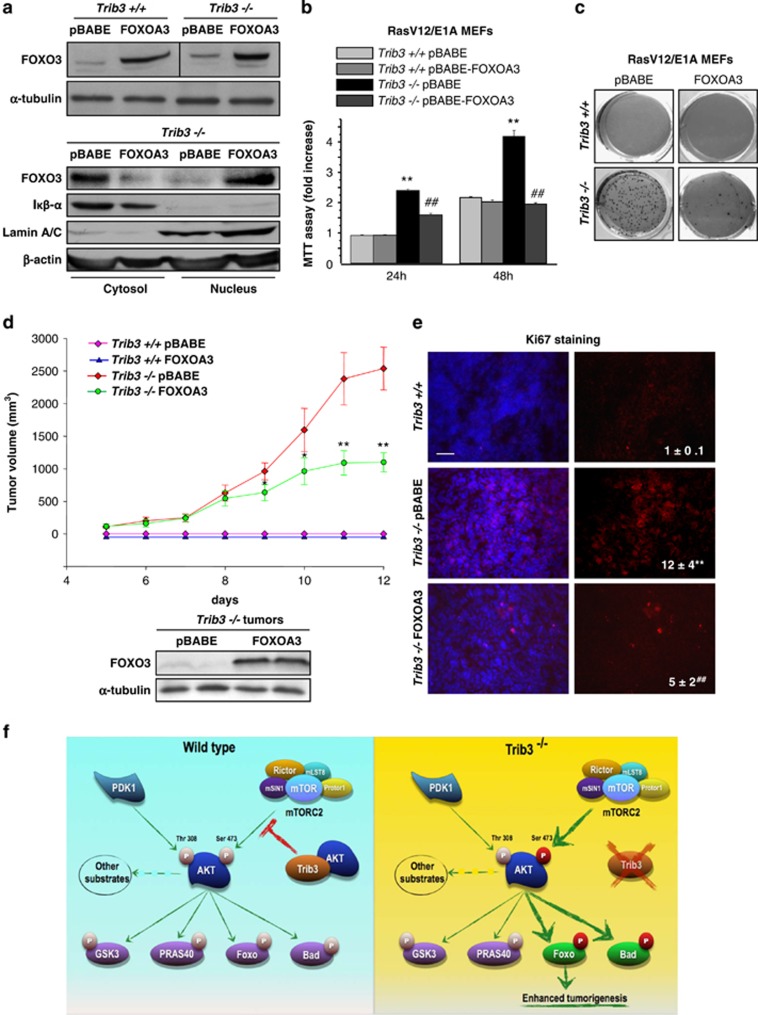
Figure 7.
Loss of TRIB3 enhances tumor growth via FOXO inactivation. (a) Upper panel: Analysis of FOXO protein levels in RasV12/E1A-transformed Trib3+/+ and Trib3−/− MEFs stably transfected with a constitutively active version of FOXO (FOXOA3) or the corresponding empty vector (pBABE). Lower panel: Sub-cellular distribution (as determined by cellular fractionation and subsequent western blotting analysis of cytosolic and nuclear fractions) of FOXO protein in RasV12/E1A-transformed Trib3−/− MEFs stably transfected with FOXOA3. β-Actin was used as a loading control. Iκβ-α and Lamin A/B were used as markers of the cytosolic and the nuclear fractions, respectively (n=3; a representative experiment is shown). (b) Effect of TRIB3 genetic inactivation and stable expression of FOXOA3 on the number of RasV12/E1A-transformed MEFs (as estimated by the MTT test) at the indicated time points. Data are expressed as the mean fold change±S.D. relative to pBABE-Trib3+/+ cells at 24 h (mean±S.D.; n=3; **P<0.01 from pBABE-Trib3+/+ cells; ##P<0.01 from pBABE-Trib3−/− cells). (c) Effect of TRIB3 genetic inactivation and stable expression of FOXOA3 on the ability of RasV12/E1A-transformed MEFs to form colonies in soft agar (n=3. A representative experiment is shown). (d) Effect of FOXOA3 stable expression and TRIB3 genetic inactivation on the growth of tumor xenografts generated by subcutaneous injection of RasV12/E1A-transformed MEFs in nude mice. Data are expressed as the mean tumor volume±S.E.M for each experimental condition (n=8 for each condition; volume of Trib3−/− FOXOA3 cell-derived tumor is compared with that of tumors derived from Trib3−/− pBabe cells; **P<0.01 from pBABE-Trib3−/− cell-derived tumors). Lower panel: Analysis of FOXO protein levels in tumors derived from FOXOA3-Trib3−/− and pBABE-Trib3−/− cells. (e) Analysis of Ki67 immunostaining in samples from tumors derived from pBABE-Trib3+/+, pBABE-Trib3−/− and FOXOA3-Trib3−/− cells. Values in the lower right corner of microphotographs correspond to the Ki67-stained area relative to the total number of nuclei in each section and are expressed as the mean fold change relative to pBABE-Trib3+/+ tumors±S.D. (**P<0.01 from pBABE-Trib3+/+ tumors; ##P<0.01 from pBABE-Trib3−/− tumors; 18 sections for each of the 3 dissected tumors for each condition were counted). Representative microphotographs are shown. (f) Proposed model of the mechanism by which TRIB3 controls tumorigenesis. TRIB3 interacts with AKT, which regulates the phosphorylation of the kinase by the mTORC2 complex (left panel). Genetic inhibition of TRIB3 in combination with different oncogenic signals facilitates the hyperphosphorylation of AKT on Ser 473 by the mTORC2 complex and the subsequent hyperphosphorylation and inactivation of the transcription factor FOXO3 (as well as of the BH3-only protein BAD) but not of the other AKT downstream targets. The hyperphosphorylation and inactivation of FOXO is responsible – at least in part – for the enhanced tumorigenic features of TRIB3-deficient cells. See also Supplementary Figure S7 and Supplementary Table SII