Abstract
Introduction:
Unlike other prevalent diseases, obstructive sleep apnea (OSA) has no simple tool for diagnosis and therapeutic decision-making in primary healthcare. Home single-channel nasal pressure (HNP) may be an alternative to polysomnography for diagnosis but its use in therapeutic decisions has yet to be explored.
Objectives:
To ascertain whether an automatically scored HNP apnea-hypopnea index (AHI), used alone to recommend continuous positive airway pressure (CPAP) treatment, agrees with decisions made by a specialist using polysomnography and several clinical variables.
Methods:
Patients referred by primary care physicians for OSA suspicion underwent randomized polysomnography and HNP. We analyzed the total sample and both more and less symptomatic subgroups for Bland and Altman plots to explore AHI agreement; receiver operating characteristic curves to establish area under the curve (AUC) measurements for CPAP recommendation; and therapeutic decision efficacy for several HNP AHI cutoff points.
Results:
Of the 787 randomized patients, 35 (4%) were lost, 378 (48%) formed the more symptomatic and 374 (48%) the less symptomatic subgroups. AHI bias and agreement limits were 5.8 ± 39.6 for the total sample, 5.3 ± 38.7 for the more symptomatic, and 6 ± 40.2 for the less symptomatic subgroups. The AUC were 0.826 for the total sample, 0.903 for the more symptomatic, and 0.772 for the less symptomatic subgroups. In the more symptomatic subgroup, 70% of patients could be correctly treated with CPAP.
Conclusion:
Automatic home single-channel nasal pressure scoring can correctly recommend CPAP treatment in most of more symptomatic patients with OSA suspicion. Our results suggest that this device may be an interesting tool in initial OSA management for primary care physicians, although future studies in a primary care setting are necessary.
Clinical Trials Information:
Clinicaltrial.gov identifier: NCT01347398.
Citation:
Masa JF, Duran-Cantolla J, Capote F, Cabello M, Abad J, Garcia-Rio F, Ferrer A, Fortuna AM, Gonzalez-Mangado N, de la Peña M, Aizpuru F, Barbe F, Montserrat JM, Spanish Sleep Network. Efficacy of home single-channel nasal pressure for recommending continuous positive airway pressure treatment in sleep apnea. SLEEP 2015;38(1):13–21.
Keywords: Apnealink, CPAP, portable monitor, sleep apnea
INTRODUCTION
Obstructive sleep apnea (OSA) is an important public health problem because of its high prevalence (close to 25% of adult population),1 impairment of the quality of life,2 and its association with cardiovascular problems3,4 and traffic accidents.5,6
Increased knowledge of OSA by general practitioners and the general population has heightened the demand for consultations with a specialist. Unlike other prevalent diseases in adults, such as hypertension and diabetes, OSA has no rapid and easy diagnostic method. In-hospital polysomnography (PSG) is the standard method but it is time-consuming and expensive; therefore, it is difficult to cover the demand, thereby giving rise to waiting lists. A type III portable monitor (at least four channels–respiratory polygraphy) is a cheaper and more rapid alternative to PSG for OSA diagnosis, but must be scored by an expert.7–9 These difficulties lead primary care physicians to take a more passive role in OSA diagnosis and treatment, as they have to refer all patients with OSA suspicion to a specialist.
In recent years, type IV portable monitoring devices have been developed, based on single-channel nasal pressure as surrogate airflow. Studies performed with home single-channel nasal pressure (HNP) with automatic scoring compared to inhospital PSG10–16 have generally shown favorable results regarding OSA diagnosis.
OSA management, like that of other diseases, requires two processes: diagnosis and therapeutic decision (i.e., continuous positive airway pressure [CPAP] or other treatments). From a practical viewpoint, the therapeutic decision process is probably the most important of the two in the primary care area. This is a very different process from diagnosis, however, and a more complex one. It is currently handled by experts, using a wide range of variables,17 the most important being relevant clinical symptoms (i.e., sleepiness), the potential consequences of OSA (i.e., cardiovascular events), and the apnea-hypopnea index (AHI) level.
We carried out a multicenter, randomized, blinded crossover study to determine, in a large sample, whether HNP AHI cutoff points based on automatic scoring could be useful, on their own, for recommending CPAP treatment that coincided with therapeutic decisions made by a sleep specialist on the basis of a set of clinical variables and in-hospital PSG.
METHODS
Subjects
From May 2011 to June 2012 we sequentially selected patients age 18–80 y, referred for suspected OSA from primary care for pulmonary consultations in 11 tertiary hospitals. Patients who had a severe and unstable disease (i.e., tumor, respiratory or heart failures) or significant nasal obstruction, lived more than 100 km from the sleep center, were psychophysically incapable of answering questionnaires, or refused to participate in the study, were excluded. This study was approved by the ethics committees of the 11 centers. Informed consent was obtained in writing from all patients.
Protocol
All the patients underwent PSG and HNP in a random order. PSG and automatic HNP scorings were carried out independently and the technicians and physicians were blinded to any patient-identifying data, as well as to any previous results.
Home Single-Channel Nasal Pressure
Our HNP (Apnealink; Resmed: Sydney, Australia) was a portable battery-powered device that measures airflow through a nasal cannula. Prior to randomization, all the patients were taught by a technician in the hospital setting how to use the HNP device at home. The device was taken by the patients from the hospital to their home and returned to the hospital the next day. The raw data file was downloaded onto a computer by a technician in each center, and these raw data were then automatically scored by Apnealink software (version 6.01). The total number of apneas and hypopneas was divided by the recording time, excluding “invalid time” (time with a bad signal that prevented scoring). The invalid time was also determined by the Apnealink software.
PSG in Hospital
We used the American Academy of Sleep Medicine (AASM) 2007 recommendations18 regarding configuration, filters, and sample signal rates. The neurological variables were electroencephalogram, electrooculogram and electromyogram (on the chin and both legs). Flow tracing was provided by a nasal cannula and thermister and thoracoabdominal motion by piezoelectric bands. Oxygen saturation was measured with a pulse oximeter (average time among centers was between 2–4 sec). Electrocardiogram and body position were also measured. The PSG studies were analyzed manually at each participating center, according to the AASM 2007 recommendations18 and respiratory scoring according to the Spanish Sleep Network rule.19
Definitions
A valid PSG required at least 3 h of sleep time and a valid HNP had at least 3 h of recorded valid flow. An invalid recording could be repeated one more time.
For PSG, an apnea was defined as the absence of airflow (≥ 90% reduction) for ≥ 10 sec and a hypopnea as a discernible airflow or band reduction (≥ 30% and < 90%) of at least 10 sec duration with a ≥ 3% drop in oxygen saturation or final arousal.19 For HNP, apnea and hypopnea were defined in the same way, but solely on the basis of the flow channel, without any desaturation or arousal. The number of apneas and hypopneas was divided by the valid time for HNP and by sleep time for PSG.
Therapeutic Decision and Variables Studied
Therapeutic decisions (CPAP or no CPAP) for PSG were made by an expert sleep specialist for each patient, based on the same set of variables collected from each patient at baseline: age, sex, body mass index, neck circumference, systolic and diastolic blood pressure, comorbidities (included hypertension), alcohol intake, and tobacco consumption. The Epworth Sleepiness Scale was measured on the basis of the four previous weeks. For PSG: date of performing, quality of recording, repetition, recording time, sleep time, sleep periods; AHI, apnea index, obstructive apnea index, central apnea index, hypopnea index (total sleep time and sleep time in the supine position); arousal, and desaturation indexes and time with SaO2 < 90%. Patients were assessed in random order using an electronic database. Participant identification numbers for patients and other data were hidden.
In general terms, the criteria for recommending CPAP from Spanish guidelines19 were: an AHI ≥ 5 with significant clinical symptoms or previous cardiovascular disease, and AHI ≥ 30 with clinical symptoms taking on less importance.
Other variables considered but not included in the therapeutic decision set were: race, formation level, employment status and health-related quality of life assessed by the European Quality of Life Questionnaire–EuroQol 5 D—(EQ 5D).20 It is a self-administered questionnaire that measures five areas of health: mobility, self-care, pain/discomfort, usual activities, and anxiety/depression. It also adds a linear visual analogical scale (Thermometer) to assess the general health situation (0 = the worst imaginable health to 100 = the best imaginable health).
Statistical Analysis
The following analysis was performed for the total sample and, separately, for a subgroup of more symptomatic patients with a prior high clinical probability of being treated with CPAP and the subgroup integrated by the rest of patients (hereafter more and less symptomatic subgroups, respectively). The selection criteria for the more symptomatic subgroup were19: an Epworth Sleepiness Scale score ≥ 12 or previous cardiovascular disease.
We performed Bland and Altman plots between AHIs from HNP and PSG for the total sample and subgroups to explore the AHI agreement between them. We constructed receiver operating characteristic (ROC) curves for automatic HNP and therapeutic decision (CPAP or not) for the total sample and subgroups to establish the area under the curve (AUC) measurements.
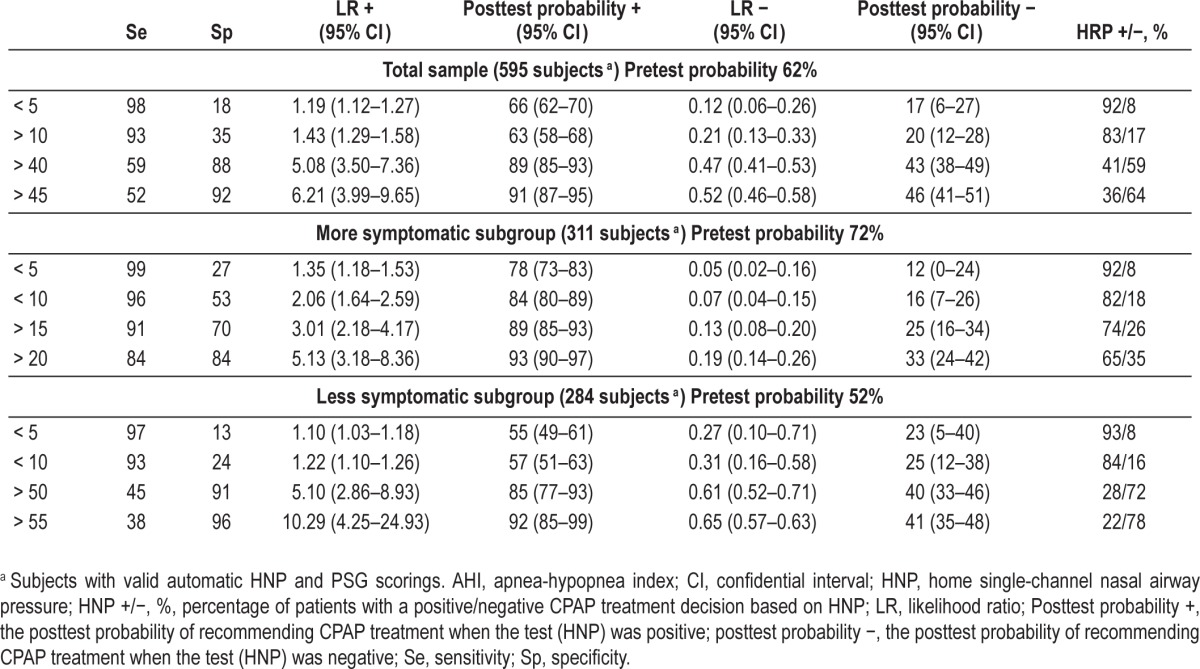
To determine the ruling-out and ruling-in HNP cutoff points for therapeutic decisions, we used: (1) sensitivity and specificity; (2) negative (1-sensitivity/specificity) and positive (sensitivity/1-specificity) likelihood ratios (LR); and (3) the posttest probability of obtaining a true positive diagnosis when the test was positive or negative, which we calculated on the basis of the pretest probability (prevalence of recommending CPAP by PSG) and positive and negative LRs.21 To find the optimal ruling-out and ruling-in HNP cutoff points, we tested the previous parameters in five-point increments of AHI (i.e., ≥ 5, ≥ 10, ≥ 15, etc.), starting with the value of five for automatic HNP scoring.
The intermediate-to-high prevalence (pretest probability) of recommending CPAP by PSG found in our study populations (62% from the total sample, 72% from the more symptomatic subgroup, and 52% from the less symptomatic subgroup) (Table 3) affects the probability of identifying an effective ruling-in HNP cutoff point, so we defined this as a positive LR > 5 with a posttest probability ≥ 90%, which represents a great change in the probability from pretest to posttest. To reduce the probability of false negatives, we also defined the ruling-out HNP cut-off point for therapeutic decision as a negative LR < 0.1 and a posttest probability ≤ 20%, which represents a great change in the probability from pretest to posttest.
Table 3.
Apnea-hypopnea index home single-channel nasal airway pressure cutoff points to rule out or rule in continuous positive airway pressure treatment in the two populations analyzed.
RESULTS
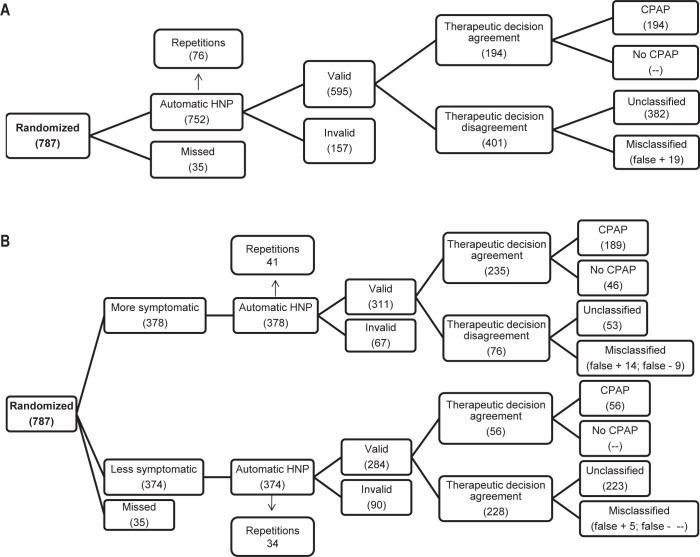
Of 877 eligible patients, 90 were excluded: 15 had severe and unstable diseases, 4 had significant nasal obstruction, 18 lived more than 100 km from the sleep center, 16 were psychophysically incapable of answering questionnaires, and 37 refused to participate in the study. Of 787 randomized patients, 35 (4%) were lost, 378 (48%) corresponded to the more symptomatic, and 374 (48%) to the less symptomatic subgroups, respectively (Figure 1).
Figure 1.
Flowchart of patients with automatic home single-channel nasal pressure (HNP) scoring, including performed and lost cases, valid and invalid recordings, and therapeutic decision agreements and disagreements. (A) For the total sample: of 787 randomized patients, 35 were lost without performing HNP and HNP was carried out in 752. Of these, 595 had valid automatic HNP and polysomnography (PSG) recordings and 157 had invalid recordings after 76 HNP repetitions. Of these 595 patients with valid recordings, 194 had therapeutic decision agreement and 401 therapeutic disagreements. All the 194 patients with therapeutic decision agreement were true positive results (continuous positive airway pressure [CPAP] recommendation). Of the 401 with therapeutic disagreement, there were 382 unclassified patients (without any CPAP recommendation) and 19 false positives. (B) For the more symptomatic and less symptomatic subgroups: of the 787 patients randomized, 35 were lost without performing HNP, 378 had a high clinical probability of being treated with CPAP and 374 did not. Of the 378 with a high clinical probability of being treated with CPAP, 311 had a valid automatic HNP scoring and 67 invalid scorings after 41 HNP repetitions. Of these 311, 235 had therapeutic decision agreement with PSG (189 with CPAP recommendation and 46 with non-CPAP recommendation) and 76 had therapeutic decision disagreement (53 unclassified–without any CPAP recommendation–14 false positives and nine false negatives). Of the 374 with no high clinical probability of being treated with CPAP, 284 had a valid automatic HNP scoring and 90 invalid scorings after 34 HNP repetitions. Of these 284, 56 had therapeutic decision agreement with PSG (all with CPAP recommendation) and 228 had therapeutic decision disagreement (223 unclassified– without any CPAP recommendation–and five false positives).
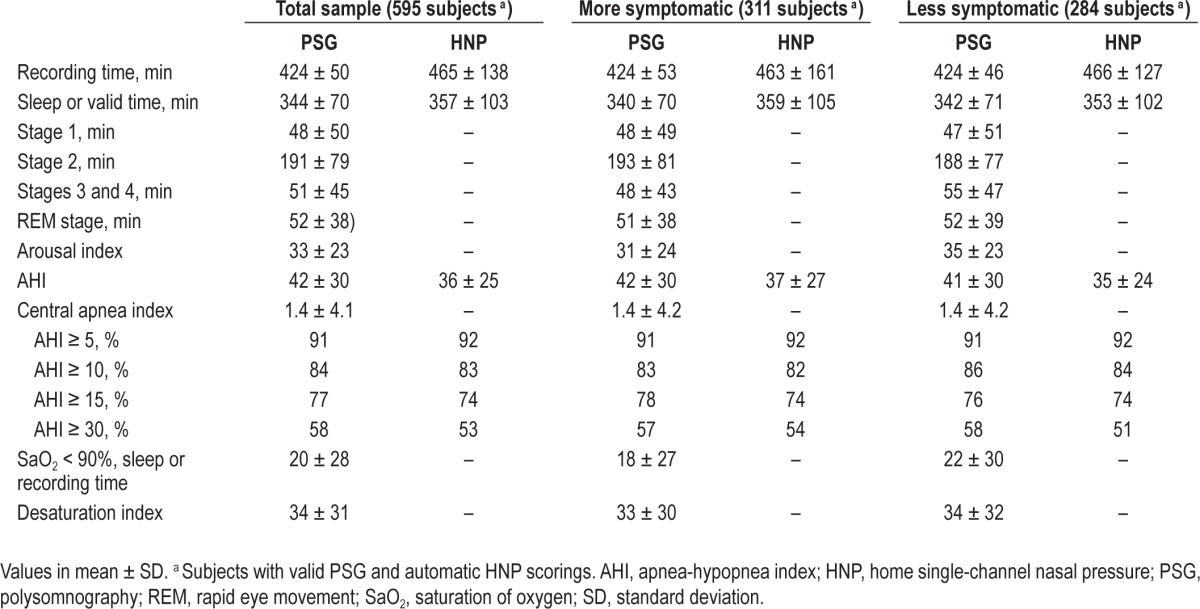
The clinical and anthropometric characteristics of the 787 randomized patients and the two subgroups are shown in Table 1. The main difference was in sleepiness scale. Higher sleep/valid recording times were observed for HNP than for PSG (Table 2). The mean AHI of the total sample was 42 ± 30 for PSG and 36 ± 25 for HNP. For the more symptomatic subgroup, it was 42 ± 30 for PSG and 37 ± 27 for HNP, and for the less symptomatic subgroup 41 ± 30 for PSG and 35 ± 24 for HNP. Lower prevalence values of OSA were observed with HNP for higher AHI, especially for the less symptomatic subgroup.
Table 1.
Characteristics of the study population.
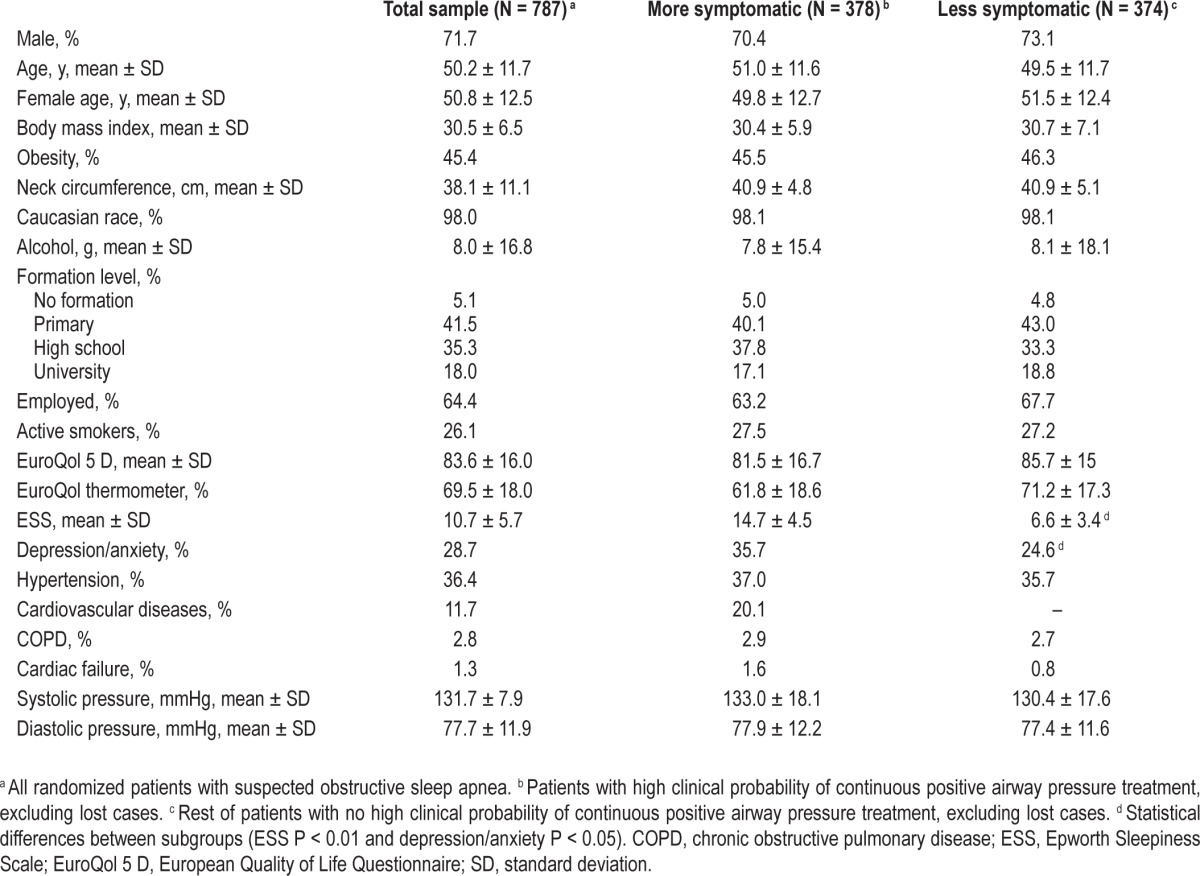
Table 2.
Sleep studies from subjects with valid polysomnography and automatic home single-channel nasal airway pressure scorings in the total sample and in the selected subgroups
The AHI agreement between PSG and HNP was moderate with high agreement limits (two standard deviations): mean of the differences = 5.8 and agreement limits = 39.6 for the total sample, 5.3 and 38.7, respectively, for the more symptomatic subgroup and 6 and 40.2, respectively, for the less symptomatic subgroup (Figure 2). The agreement was not uniform across the AHI level. It was worse for intermediate and higher AHI values for the total sample and particularly worse for intermediate AHI values (i.e., from 15 to 40) in the less symptomatic subgroup (see Discussion).
Figure 2.
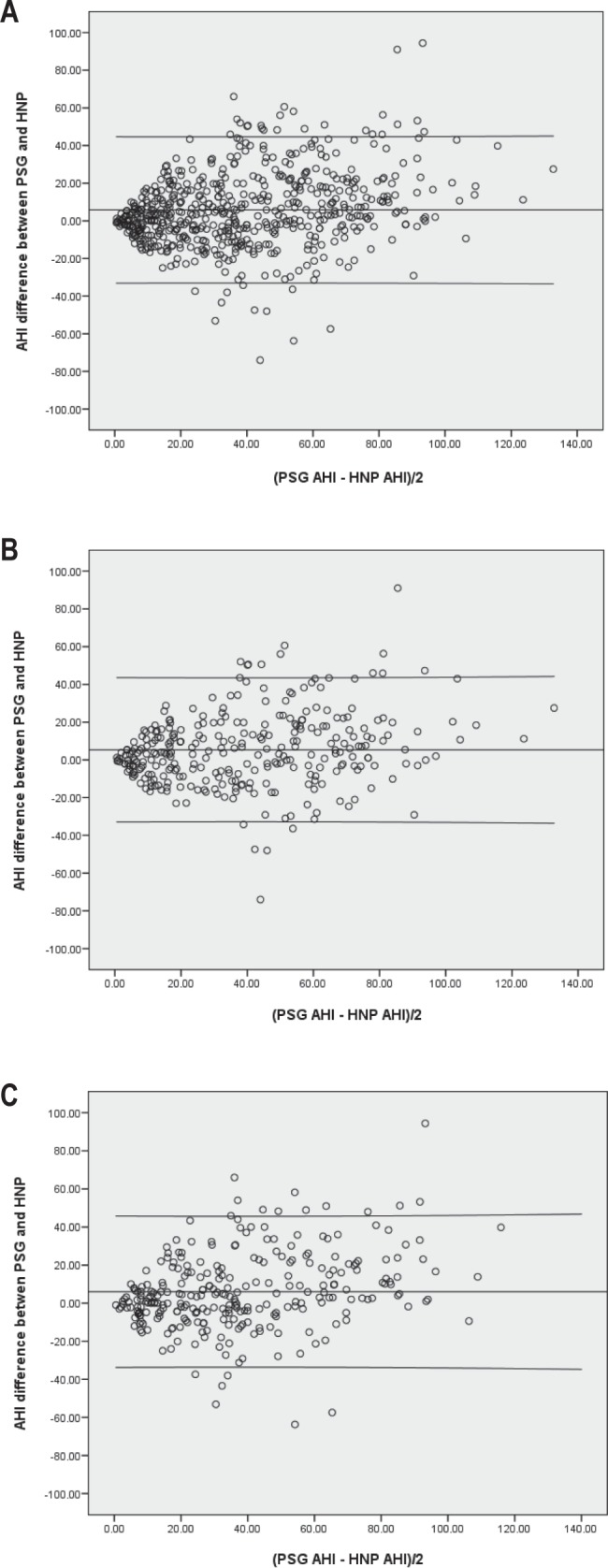
Mean AHI versus the difference in AHI between PSG and automatic HNP scorings (Bland-Altman plots). Central lines represent mean values and upper and lower lines represent agreement limits (two standard deviations). (A) entire sample, (B) more symptomatic subgroup and (C) less symptomatic subgroup. AHI, apnea-hypopnea index; HNP, home single-channel nasal airway pressure; PSG, polysomnography.
Figure 3 shows the ROC curves for HNP scoring and the therapeutic decision (CPAP or not) in the total sample and both subgroups. The AUC from the total sample and the less symptomatic subgroup were clearly lower than that of the more symptomatic sub-sample.
Figure 3.
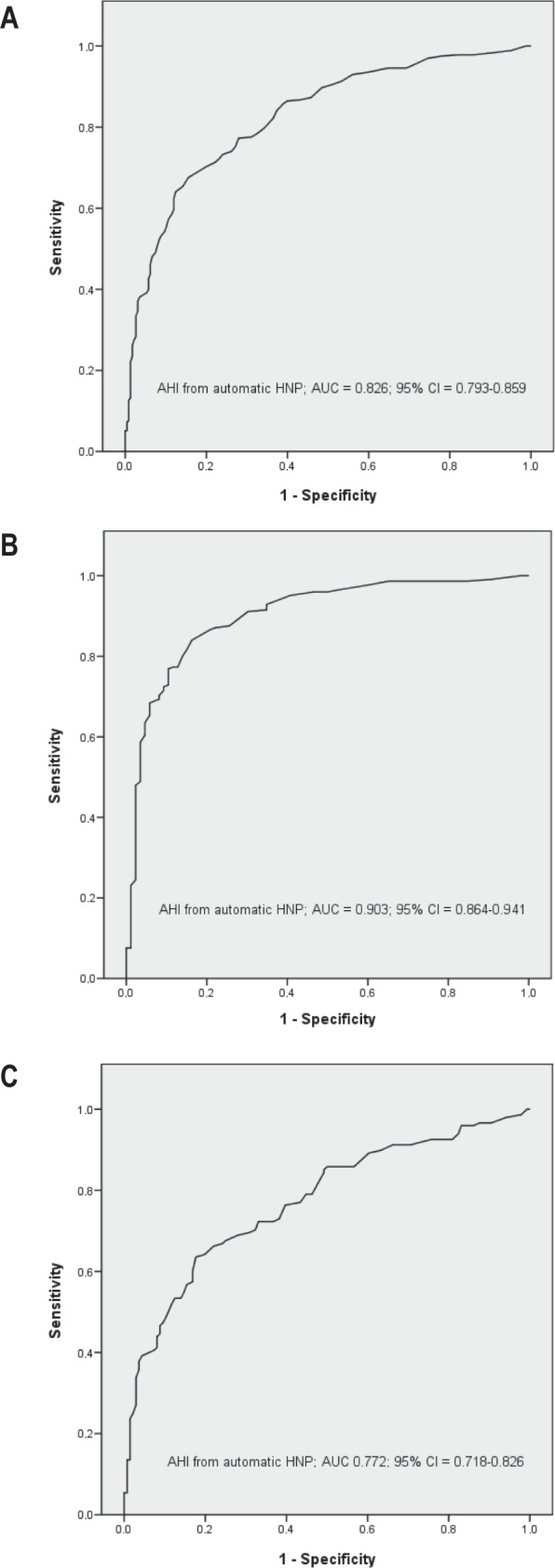
Receiver operating characteristic (ROC) curves for automatic HNP scorings based on CPAP recommendation (yes or no) by PSG. (A) entire sample, (B) more symptomatic subgroup, (C) less symptomatic subgroup. AHI, apnea-hypopnea index; AUC, area under the curve; CI, confidence interval; CPAP, continuous positive airway pressure; HNP, home single-channel nasal airway pressure; PSG, polysomnography.
Of the 787 randomized patients, HNP was performed in 752 (Figure 1A). Of these, 595 had valid automatic HNP and PSG recordings (79%) after 76 HNP repetitions. Of these 595, the pretest probability (prevalence of CPAP treatment) was 62% (Table 3). According to our previous definition (see Statistical Analysis), there was no HNP AHI cutoff point for effectively ruling out CPAP treatment. A suitable HNP AHI cutoff point for ruling in CPAP treatment would be ≥ 45. Therefore, there were 194 patients with therapeutic decision agreement (33% of patients with valid scorings) and almost double (401) with therapeutic disagreement (Figure 1A). All the 194 patients with therapeutic decision agreement represented true positive results (CPAP recommendation). Of the 401 with therapeutic disagreement, there were 382 unclassified patients (without CPAP recommendation) (64% of patients with valid scorings) and 19 false positives (3% of patients with valid scorings).
Of the 378 more symptomatic patients (Figure 1B), 311 had valid automatic HNP and PSG recordings (82%) after 41 HNP repetitions. Of these 311, the pretest probability (prevalence of CPAP treatment) was 72% (Table 3). According to our previous definition (see Statistical Analysis), an HNP AHI cutoff point ≤ 10 effectively ruled out CPAP treatment. An acceptable HNP AHI cutoff point for ruling in CPAP treatment would be ≥ 20. Therefore, there were 235 patients with therapeutic decision agreement (76% of patients with valid scorings) and 76 with therapeutic disagreement (Figure 1). Of these 235, there were 46 patients with a true negative result (no CPAP recommendation) and 189 with a true positive result (CPAP recommendation). Of the 76, there were 53 unclassified patients (without any CPAP recommendation) (17% of patients with valid scorings) and 14 false positives (4% of patients with valid scorings) and nine false negatives (3% of patients with valid scorings).
Of the 374 less symptomatic patients (Figure 1B), 284 had valid automatic HNP and PSG recordings (76%) after 34 HNP repetitions. Of these 284, the pretest probability (prevalence of CPAP treatment) was 52% (Table 3). According to our previous definition (see Statistical Analysis), there was no HNP AHI cutoff point for effectively ruling out CPAP treatment. A HNP AHI cutoff point ≥ 55 could rule in CPAP treatment. Therefore, there were only 56 patients with therapeutic decision agreement (20% of patients with valid scorings), and 228 with therapeutic disagreement (Figure 1B). Of these 228, there were 56 with a true positive result (CPAP recommendation), 223 unclassified patients (without any CPAP recommendation) (79% of patients with valid scorings) and five false positives (2% of patients with valid scorings).
DISCUSSION
To our knowledge, this study is the first to explore the usefulness of HNP AHI cutoff points for recommending CPAP treatment that agrees with a therapeutic decision made by a sleep specialist using PSG. The main results were: (1) automatic HNP scoring can effectively exclude and confirm CPAP recommendation in agreement with PSG only in the subgroup of more symptomatic patients; (2) most of these selected patients can be assigned to CPAP or non-CPAP treatment; the remaining patients will need other more specialized management.
The therapeutic decision (CPAP or not) is a complex process based on many variables and it is undertaken by specialized physicians. Therefore, when a primary care physician is suspicious for OSA, it is important to establish the diagnosis but also, more particularly, to identify which patients need a rapid application of CPAP treatment.
Our results suggest a potential application into the field of primary care. The patients included in our study were directly selected by primary care practitioners on the basis of OSA suspicion. These physicians could therefore select more symptomatic patients (Epworth Sleepiness Scale ≥ 12 or cardiovascular problems, approximately 50% of patients with OSA suspicion) for HNP. After valid automatic scoring, patients with HNP AHI < 10 would not need CPAP treatment and could be treated with hygienic-dietary measures until they attended a subsequent, more specialized consultation. Patients with HNP AHI ≥ 20 could be treated with CPAP. The optimal pressure could be calculated by an AutoCPAP device22 used in primary healthcare or a tertiary hospital, depending on the level of training of the primary care physicians. A minority of these more symptomatic patients (approximately 30%) would have an invalid automatic scoring or no effective therapeutic decision (unclassified patients), and they should be referred preferentially for specialist consultation. A small proportion of false positives (4%) and negatives (3%) should be assumed, although most of these would be borderline cases for CPAP decision. Including these false positives and negatives, 68% of more symptomatic patients could be initially managed in primary healthcare and 70% of the 72% of candidates could be treated correctly with CPAP, according to the expert decision.
A recent study23 has evaluated the efficacy of a program of management of diagnosis and treatment by trained primary care physicians using oximetry versus habitual specialized in-hospital management using PSG. Patients were selected by having intermediate to high clinical probability of sleep apnea. Clinical efficacy was similar in both groups at the end of the 6 mo of follow-up but primary care physicians chose CPAP for 90% of patients while specialists use it for 70% of patients. In the primary care group, 27% of patients discontinued CPAP and only 9% in the hospital group. These data may indicate that therapeutic CPAP decision is a very complicated process even for trained primary care physicians. Our proposal takes the advantage that the therapeutic decision is included in the proposal simplifying the primary care training and management. However, a specific validation of our protocol in primary care setting is necessary.
In the less symptomatic subgroup, there was a nonnegligible percentage of patient candidates for CPAP treatment (52%). However, using HNP only for more symptomatic patients can have the following advantages: saving the cost of HNP and inconvenience in half the patients; more rapid therapeutic decisions in the group of patients with a greater capacity to improve; and these more symptomatic patients could have better adherence to CPAP than less symptomatic patients, favoring easier management for nonexpert physicians.
Some studies have proposed performing HNP for all patients with OSA suspicion and recommending CPAP for severe cases on the basis of the AHI (i.e. HNP AHI ≥ 30).10,12,15,16 The problems with this approach are the underestimation of HNP AHI in comparison with PSG and the high variability between the two methods (Figure 2), especially for intermediate-to-high AHI values. These factors make it difficult to achieve a cutoff point for HNP AHI alone that rules in or rules out OSA, or a broad unclassified population when different cutoff points for ruling in and ruling out OSA are evaluated (i.e., from 21 to 59).10 In our population, the AUC between a therapeutic decision by an expert using PSG and automatic HNP in patients with automatic HNP AHI ≥ 30 and < 30 were lower (0.749 and 0.684, respectively) than in the more symptomatic subgroup (0.903) and the less symptomatic subgroup (0.772). Therefore, there is better agreement in therapeutic decision selecting more symptomatic patients than selecting patients with an HNP AHI ≥ 30.
Five randomized controlled studies have evaluated the efficacy of CPAP treatment after OSA diagnosis using PSG or other types of portable home monitoring devices.24–28 Patients with a high clinical probability of OSA were selected to receive CPAP treatment if OSA was established. Both protocols (home portable monitor and in-hospital PSG) showed similar improvements in AHI,26 quality of life, clinical symptoms, and adherence to CPAP treatment.24,25,27,28 Most of these studies24–27 recommend CPAP for an AHI ≥ 15 with both diagnostic methods. Our study confirms that even in patients selected for their high clinical probability of OSA, an intermediate level of AHI (i.e., ≥ 20) is required to achieve satisfactory agreement with a CPAP recommendation carried out by an expert on the basis of PSG. This result is also similar to another large study assessing the agreement in the therapeutic decision (CPAP or non-CPAP) performed by home respiratory polygraphy (a type III device) or by in- hospital PSG.17
Twenty-one percent of patients who performed automatic HNP (12% for more symptomatic and 24% for less symptomatic subgroups) had invalid recording because of insufficient time (< 180 min). Some HNP devices have an additional channel with oxygen saturation,11,12 although a separate analysis did not increase the diagnostic efficacy in patients with OSA suspicion. Nevertheless, we wonder if by using an additional oxygen saturation scoring we would recuperate some invalid cases. It could be an interesting approach for future studies.
We evaluated an automatic HNP scoring to explore the therapeutic decision instead of manual scoring. Manual scoring has slightly better agreement with the PSG than automatic scoring for OSA diagnosis.29 However, we performed the current study as the first step to apply the results in an ulterior study comparing the OSA management efficacy between primary care and hospital sites. Manual scoring requires special training that is more difficult to achieve in the primary care area.
As previously discussed, the AHI agreement between HNP and PSG was moderate, the same as in other similar studies.7 This could be caused by longer recording times with HNP in comparison with PSG sleep time and derivative factors of performing PSG and HNP on different nights such as: shorter sleep time in a supine position, night-to-night variability, and better sleep quality and stability.
As a result of our clinical trial design, severe and unstable diseases, such as some chronic heart failure with high probability of developing central apneas, were excluded. We had no patients with Cheyne-Stokes respiration; furthermore, the PSG central apnea index was very low (Table 2), minimizing the fact that HNP cannot identify central events.
Because OSA is a very prevalent disease, it must be managed by different levels of health attention, as in the cases of other prevalent diseases such as asthma, hypertension and diabetes. If primary care physicians are to become more involved in OSA management, they need a tool for diagnosis and therapeutic decisions, as well as an integrated network that defines the roles of both primary and specialist practitioners. Our study evaluates a tool for deciding whether or not to recommend CPAP treatment for more symptomatic patients, thereby helping non-experts to use an expert device.
In conclusion, automatic HNP scoring can correctly recommend CPAP treatment in most more symptomatic patients suspected of having OSA. This device could be an interesting tool for the initial management of OSA for primary care physicians working with specialists within an integrated network. Future studies performed in a primary care setting should confirm this result.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was funded by the Instituto de Salud Carlos III (Fondo de Investigaciones Sanitarias, Ministerio de Sanidad y Consumo) PI050402, Spanish Respiratory Foundation 2005 (FEPAR) and Departamento de Sanidad del Gobierno Vasco (2005111010) and Caja Vital (2005).The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are indebted to Verónica Rodríguez and Vanessa Iglesias for their assistance in the translation of the manuscript. We also thank Almudena Fernández, Joaquin D. Carro and Carmen González for their help and technical assistance during the study.
Footnotes
A commentary on this article appears in this issue on page 7.
REFERENCES:
- 1.Durán J, Esnaola S, Rubio R, et al. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163:685–9. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 2.Ballester E, Badia JR, Hernández L, et al. Evidence of the effectiveness of continuous positive airway pressure in the treatment of sleep apnea/ hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:495–501. doi: 10.1164/ajrccm.159.2.9804061. [DOI] [PubMed] [Google Scholar]
- 3.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 4.Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, et al. Spanish Sleep and Breathing Network. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307:2161–8. doi: 10.1001/jama.2012.4366. [DOI] [PubMed] [Google Scholar]
- 5.Terán-Santos J, Jiménez-Gómez A, Cordero-Guevara J, et al. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-Santander. N Engl J Med. 1999;340:847–51. doi: 10.1056/NEJM199903183401104. [DOI] [PubMed] [Google Scholar]
- 6.Masa JF, Rubio M, Findley LJ, et al. Habitually sleepy drivers have a high frequency of automobile crashes associated with respiratory disorders during sleep. Am J Respir Crit Care Med. 2000;162:1407–12. doi: 10.1164/ajrccm.162.4.9907019. [DOI] [PubMed] [Google Scholar]
- 7.Masa JF, Corral J, Pereira R, et al. Effectiveness of home respiratory polygraphy for the diagnosis of sleep apnoea and hypopnoea syndrome. Thorax. 2011;66:567–73. doi: 10.1136/thx.2010.152272. [DOI] [PubMed] [Google Scholar]
- 8.Flemons WW, Littner MR, Rowley JA, et al. Home diagnosis of sleep apnea: a systematic review of the literature. An evidence review cosponsored by the American Academy of Sleep Medicine, the American College of Chest Physicians, and the American Thoracic Society. Chest. 2003;124:1543–79. doi: 10.1378/chest.124.4.1543. [DOI] [PubMed] [Google Scholar]
- 9.Collop NA, Anderson WM, Boehlecke B, et al. Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–47. [PMC free article] [PubMed] [Google Scholar]
- 10.Wong KHK, Jankelson D, Reid A, et al. Diagnostic test evaluation of a nasal flow monitor for obstructive sleep apnea detection in sleep apnea research. Behav Res Methods. 2008;40:360–6. doi: 10.3758/brm.40.1.360. [DOI] [PubMed] [Google Scholar]
- 11.Ragette R, Wang Y, Weinreich G, et al. Diagnostic performance of single airflow channel recording (ApneaLink) in home diagnosis of sleep apnea. Sleep Breath. 2010;14:109–14. doi: 10.1007/s11325-009-0290-2. [DOI] [PubMed] [Google Scholar]
- 12.Rofail LM, Wong KK, Unger G, et al. Comparison between a single-channel nasal airflow device and oximetry for the diagnosis of obstructive sleep apnea. Sleep. 2010;33:1106–14. doi: 10.1093/sleep/33.8.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watkins MR, Talmage JB, Thiese MS, et al. Correlation between screening for obstructive sleep apnea using a portable device versus polysomnography testing in a commercial driving population. J Occup Environ Med. 2009;51:1145–50. doi: 10.1097/JOM.0b013e3181b68d52. [DOI] [PubMed] [Google Scholar]
- 14.Erman MK, Stewart D, Einhorn D, et al. Validation of the ApneaLink for the screening of sleep apnea: a novel and simple single-channel recording device. J Clin Sleep Med. 2007;3:387–92. [PMC free article] [PubMed] [Google Scholar]
- 15.Rofail LM, Wong KK, Unger G, et al. The utility of single-channel nasal airflow pressure transducer in the diagnosis of OSA at home. Sleep. 2010;33:1097–105. doi: 10.1093/sleep/33.8.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oktay B, Rice TB, Atwood CW, Jr, et al. Evaluation of a single-channel portable monitor for the diagnosis of obstructive sleep apnea. J Clin Sleep Med. 2011;7:384–90. doi: 10.5664/JCSM.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masa JF, Corral J, Pereira R, et al. Spanish Sleep Network. Therapeutic decision-making for sleep apnea and hypopnea syndrome using home respiratory polygraphy: a large multicentric study. Am J Respir Crit Care Med. 2011;184:964–71. doi: 10.1164/rccm.201103-0428OC. [DOI] [PubMed] [Google Scholar]
- 18.Iber C, Ancoli-Israel S, Chesson A, et al. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 19.Grupo Español de Sueño (GES) Consenso Nacional sobre el síndrome de apneas-hipopneas del sueño. Arch Bronconeumol. 2005;41:3–110. [Google Scholar]
- 20.EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 21.Flemons WW, Littner MR. Measuring agreement between diagnostic devices. Chest. 2003;124:1535–42. doi: 10.1378/chest.124.4.1535. [DOI] [PubMed] [Google Scholar]
- 22.Masa JF, Jiménez A, Durán J, et al. Alternative methods of titrating continuous positive airway pressure: a large multicenter study. Am J Respir Crit Care Med. 2004;170:1218–24. doi: 10.1164/rccm.200312-1787OC. [DOI] [PubMed] [Google Scholar]
- 23.Chai-Coetzer CL, Antic NA, Rowland LS, et al. Primary care vs specialist sleep center management of obstructive sleep apnea and daytime sleepiness and quality of life: a randomized trial. JAMA. 2013;309:997–1004. doi: 10.1001/jama.2013.1823. [DOI] [PubMed] [Google Scholar]
- 24.Kuna ST, Gurubhagavatula I, Maislin G, et al. Non-inferiority of Functional Outcome in Ambulatory Management of Obstructive Sleep Apnea. Am J Respir Crit Care Med. 2011;183:1238–44. doi: 10.1164/rccm.201011-1770OC. [DOI] [PubMed] [Google Scholar]
- 25.Rosen CL, Auckley D, Benca R, et al. A multisite randomized trial of portable sleep studies and positive airway pressure autotitration versus laboratory-based polysomnography for the diagnosis and treatment of obstructive sleep apnea: the HomePAP study. Sleep. 2012;35:757–67. doi: 10.5665/sleep.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulgrew AT, Fox N, Ayas NT, et al. Diagnosis and initial management of obstructive sleep apnea without polysomnography: a randomized validation study. Ann Intern Med. 2007;146:157–66. doi: 10.7326/0003-4819-146-3-200702060-00004. [DOI] [PubMed] [Google Scholar]
- 27.Antic NA, Buchan C, Esterman A, et al. A randomized controlled trial of nurse-led care for symptomatic moderate-severe obstructive sleep apnea. Am J Respir Crit Care Med. 2009;179:501–8. doi: 10.1164/rccm.200810-1558OC. [DOI] [PubMed] [Google Scholar]
- 28.Berry RB, Hill G, Thompson L, et al. Portable monitoring and autotitration versus polysomnography for the diagnosis and treatment of sleep apnea. Sleep. 2008;31:1423–31. [PMC free article] [PubMed] [Google Scholar]
- 29.Nigro CA, Dibur E, Malnis S, Grandval S, Nogueira F. Validation of ApneaLink Ox™ for the diagnosis of obstructive sleep apnea. Sleep Breath. 2013;17:259–66. doi: 10.1007/s11325-012-0684-4. [DOI] [PubMed] [Google Scholar]