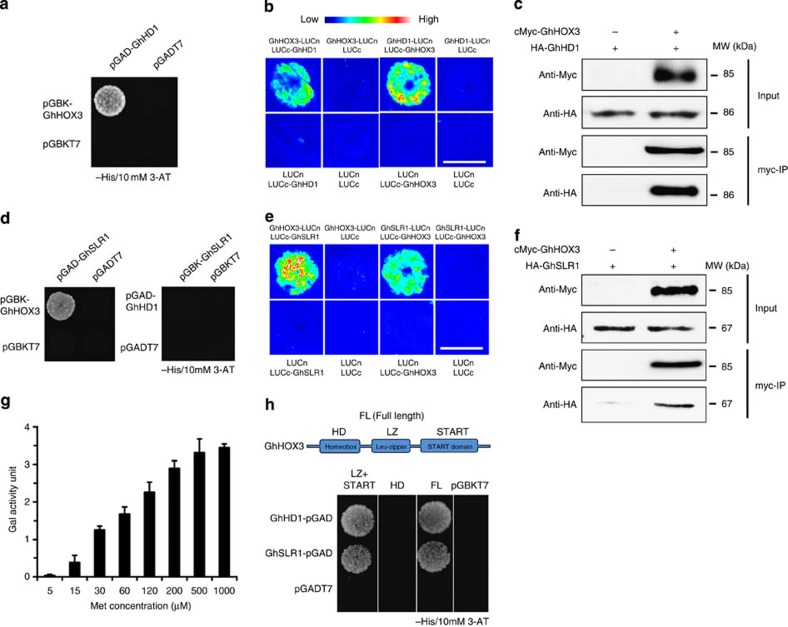
Figure 3. The DELLA protein GhSLR1 binds to GhHOX3 and interferes with the GhHOX3–GhHD1 interaction.
(a) Yeast two-hybrid assay. pGAD-GhHD1 combined with pGBK-GhHOX3 conferred yeast growth on SD/-Leu/-Trp/-His plates supplemented with 10 mM 3-amino-1,2,4-triazole (3-AT). (b) BiFc assay. GhHOX3 and GhHD1 were interchangeably fused to the carboxyl- and amino-terminal of firefly luciferase (LUC, LUCc and LUCn), transiently co-expressed, and LUCc or LUCn was co-expressed with each other or with each un-fused target protein as the control. Fluorescence signal intensities represent their binding activities. Top bar, heat map’s scale of the signal intensity. GhHOX3 interacted with GhHD1. Scale bar, 1 cm in b. (c) Coimmunoprecipition (CoIP) of transiently co-expressed cMyc-GhHOX3 and HA-GhHD1 in leaves of Nicotiana benthamiana. Soluble protein extracts before (input) and after (IP) immunoprecipitation with anti-cMyc antibody-conjugated beads were detected by immunoblot with anti-HA antibody. (d) Yeast two-hybrid assay. GhHOX3, but not GhHD1, bound to GhSLR1 at 10 mM 3-AT. (e,f) In vivo BiFc (e) and CoIP (f) assays. GhHOX3 interacted with GhSLR1. Scale bar, 1 cm in e. (g) Yeast three-hybrid assay showing the influence of GhSLR1 on GhHOX3–GhHD1 binding represented by β-galactosidase activity, and the GhSLR1 expression was suppressed by increasing Met concentrations (data are shown as mean±s.e.m., n=3). (h) Domain deletion assay. Top, GhHOX3 contains three conserved domains. Below, yeast two-hybrid detection. GhHOX3 fragment containing both the Leu-zipper (LZ) and the START domains interacted with both GhHD1 and GhSLR1 in yeast, whereas the GhHOX3 homeodomain (HD) did not.