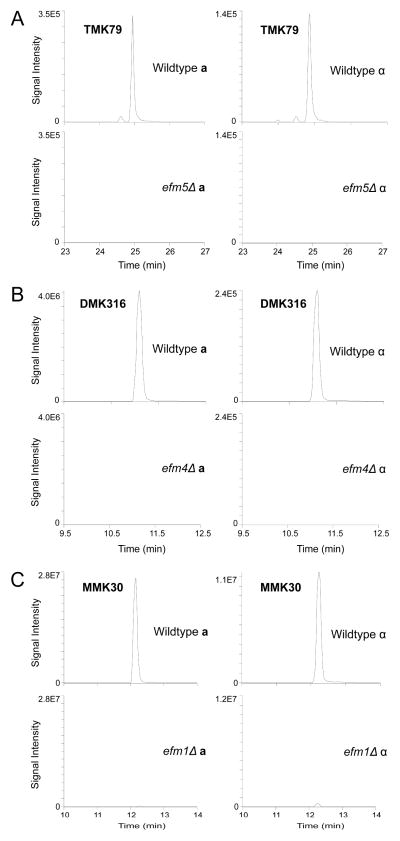
Figure 2. Deletion of EFM5 results in loss of trimethyllysine 79 on elongation factor 1A.
The 50 kDa protein bands from wildtype and knockout lysates were excised, in-gel trypsin digested, and analyzed by LC-MS/MS. Extracted ion chromatograms correlating to the EF1A methylated peptides (precursor → product ion transition) from wildtype and each EFM knockout strain are shown. MS/MS data of individual peptides is shown in Supplemental Figure S2. (A) Loss of EF1A trimethylation on peptide GITIDIALWKtrimethylFETPK in efm5Δ was visualized by loss of the 592.010 m/z → 791.466 m/z transition. (B) Loss of EF1A dimethylation on NVSVKdimethylEIR in efm4Δ was visualized by loss of the 324.866 m/z → 573.371 m/z transition. (C) Loss of EF1A monomethylation on peptide STTTGHLIYKmonomethyl in efm1Δ was visualized by loss of the 567.811 m/z → 744.440 m/z transition.