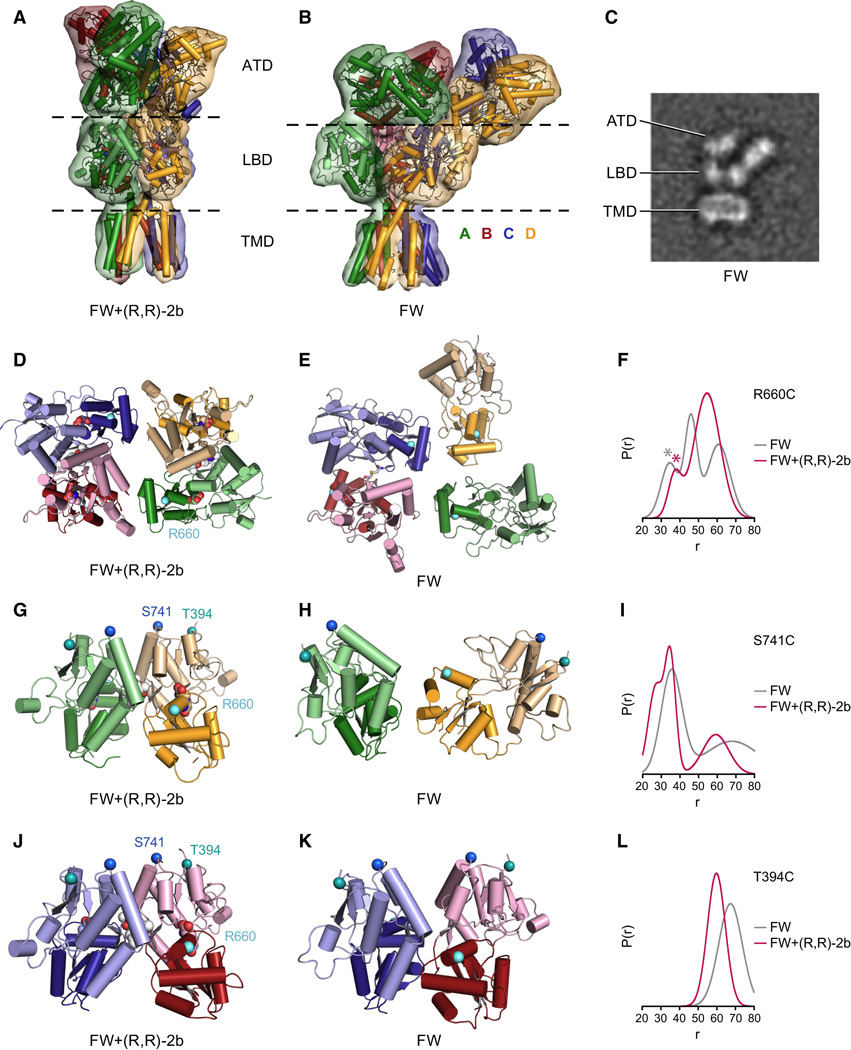
Figure 5. Conformational rearrangements upon receptor desensitization.
Comparison of the non-desensitized full-length structure in complex with FW and (R,R)-2b (A,D,G,J) and low resolution X-ray (B,E,H,K) or cryo-EM (C) structures complexed with FW alone. A. X-ray structure in complex with FW+(R,R)-2b. B. Molecular replacement solution obtained from an 8.0 Å dataset collected from a crystal grown in presence of FW. C. Selected cryo-EM class average of the same receptor construct/ligand combination as in B (see Figure S5 for all class averages). The side length of the panel is 29.1 nm. D,E. View of LBD layers from the top of the receptor, Cα atoms of Arg 660 are shown as white spheres. In panel E, the disulfide link between S729C side chains in chains B and C is shown in yellow stick representation. F. Distance distributions of MTSL-labeled R660C receptor measured with FW (grey) or FW+(R,R)-2b (magenta). G,H. Side view of LBD dimer AD. Cα atoms of Ser 741 are shown as blue spheres, Cα atoms of Thr 394 are shown as teal spheres. I. Distance distributions of MTSSL-labeled S741C receptor measured with FW (grey) or FW+(R,R)-2b (magenta). J,K. Side view of LBD dimer BC. Cα atoms of Ser 741 are shown as blue spheres, Cα atoms of Thr 394 are shown as teal spheres. L. Distance distributions of MTSSL-labeled T394C receptor measured with FW (grey) or FW+(R,R)-2b (magenta). DEER decays of the shown mutants R660C, S741C and T394C are provided in Figure S5 C–E). See also Figure S5. Movie M4 and Movie M5.