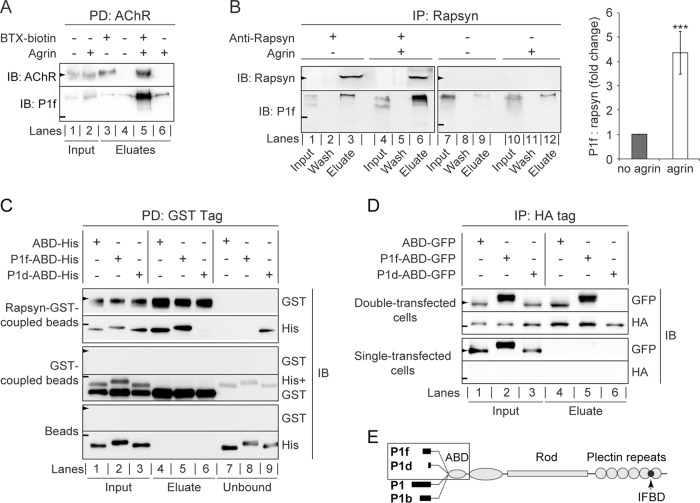
FIGURE 5:
Targeting of P1f to the AChR complex via binding to rapsyn. (A) Copurification of P1f with sarcolemma-associated AChRs. Membrane-bound AChRs were isolated from untreated or agrin-treated C2C12 myotubes by incubation of cells with biotinylated α-BTX, followed by cell lysis and precipitation of the biotinylated AChR using streptavidin-agarose. Input (lysates) and eluate fractions were analyzed by immunoblotting (IB). Lysates from BTX-untreated cells served as negative controls (lanes 4 and 6). (B) Coimmunoprecipitation of P1f with rapsyn from lysates of untreated/agrin-treated C2C12 myotubes (input) using anti-rapsyn antibodies and protein G–agarose. Wash and eluate fractions of precipitates were subjected to immunoblotting (IB) as indicated. Note that the levels of endogenous rapsyn are very low and become detectable only upon immunoprecipitation of the protein. The bar diagram shows signal intensities of P1f, densitometrically determined and normalized to coeluted rapsyn (P1f/rapsyn = 1, for agrin-untreated cells). Note the greater than fourfold higher amount of P1f cosedimenting with rapsyn from agrin-treated C2C12 myotubes compared to control (no agrin). Mean value ± SEM, three experiments. ***p < 0.001 compared with no-agrin control. (C) GST pull-down assay. Top two rows, recombinant rapsyn-GST coupled to glutathione–Sepharose beads was incubated with His-tagged versions of plectin's ABD, P1f-ABD, or P1d-ABD, and rapsyn-bound/unbound fractions were separated by centrifugation. Aliquots of input (lanes 1–3), bound (eluates; lanes 4–6), and unbound fractions (lanes 7–9) were analyzed by immunoblotting (IB) using antibodies to protein tags as indicated. Middle two and lower two rows, like the top two rows, except that only recombinant GST protein (without fused rapsyn) or no protein at all, respectively, was immobilized on glutathione–Sepharose beads. Note that P1f-ABD, as well as ABD, is coeluting with rapsyn-GST (second row, lanes 4 and 5), whereas P1d-ABD is predominantly detected in the unbound fraction (lane 9). (D) Pull down of N-terminal plectin fragments with rapsyn overexpressed in HEK cells. Top two rows, lysates derived from HEK-cells cotransfected with rapsyn-HA and GFP-tagged plectin fragments were subjected to immunoprecipitation using HA-specific antibodies and protein G–coupled agarose beads; input and eluate fractions were analyzed as in to D. Note the binding of P1f-ABD and ABD (lanes 4 and 5), but not of P1d-ABD (lane 6), to rapsyn-HA. Bottom two rows, like the upper two rows, except that HEK cells were single transfected (overexpressing plectin fragments but not rapsyn). (E) Schematic representation of the domain structure of the four major plectin isoforms (P1f, P1d, P1, and P1b) expressed in skeletal muscle. Recombinant His/GFP-tagged N-terminal domains of isoforms used for binding assays and coimmunoprecipitation are boxed. Arrowheads and short lines on the left in A–D mark positions of molecular mass markers (in kilodaltons): A and B, 43 and 250; C, 70 and 35; D, 55 and 43 (arrowheads and lines, respectively).