Abstract
Restrictive ventricular septal defect (rVSD) presents with little/no hemodynamic aberrations despite a patent septal defect. Clinically, these patients are observed with the hope that the defect will functionally close over time without the need for surgical repair and development of heart failure. Without evidence supporting a definitive therapeutic strategy, rVSD patients may have increased risk of a poor outcome. We tested the hypothesis that rVSD results in subclinical RV diastolic dysfunction and molecular remodeling. Five pigs underwent surgical rVSD creation. Echocardiography, hemodynamics, myocyte contractility experiments, and proteomics/Western blot were performed 6-weeks post-rVSD and in controls. *p < 0.05. LV and RV hemodynamics in rVSD were comparable to controls. The tricuspid valve early/late diastolic inflow velocity ratio (TV E/A ratio) decreased from 1.6 ± 0.05 in controls to 1.0 ± 0.08* in rVSD, indicating RV diastolic dysfunction. rVSD RV myocytes showed abnormalities in contraction (departure velocity (Vd) − 51%*, Vd time +55%*) and relaxation (return velocity (Vr) −50%*, Vr time +62%*). Mitochondrial proteins (fatty acid, TCA cycle) increased 2-fold*, indicating heightened RV work. Desmin protein upregulated 285%* in rVSD RV myocardium, suggesting cytoskeletal remodeling. rVSD causes RV diastolic dysfunction, myocyte functional impairment, and mitochondrial/cytoskeletal protein upregulation in our model. Desmin upregulation may hinder sarcomeric organization/relaxation, representing a key subclinical early marker for future RV dysfunction. TV E/A measurements are a non-invasive modality to assess rVSD patients for diastolic dysfunction. Translational research applications may lead to fundamental changes in the clinical management of rVSD by providing evidence for early repair of the defect.
Keywords: Ventricular septal defect, Diastolic dysfunction, Desmin, Remodeling, Congenital, Cytoskeleton, Echocardiography
1. Introduction
Ventricular septal defect (VSD) is a congenital cardiac abnormality defined by a hole in the ventricular septum which enables blood to shunt from the left ventricle (LV) to the right ventricle (RV). VSD is the most common congenital heart defect, comprising 15–30% of all abnormalities and occurring in 1–3/1000 live births and 5–10% of spontaneous abortions [1,2]. Muscular VSD (as opposed to perimembranous, supracristal, and inlet VSDs) results from failure of the septum to completely close during cardiac development. The majority of VSDs are sporadic and the underlying mechanism is most likely multigenic [2]. Large VSDs (nonrestrictive) result in high ventricular pressure gradients and significant blood shunting, leading to pulmonary hypertension, RV dysfunction, and intractable heart failure (HF) if left untreated. Smaller VSDs, in which shunt flow is occluded via septal compression during systole, are called “restrictive VSD” (rVSD).
Medical management of large VSDs is straightforward: the defect must be closed or the patient will develop pulmonary hypertension and RV failure. Management of rVSD is less clear: rVSD presents with little/no hemodynamic aberrations despite a patent septal defect and the presence of a left-to-right shunt. Controversy exists as to how the risk of surgical closure in an asymptomatic patient weighs against the lifetime risk of a non-operated rVSD. Clinically, these patients are observed with the hope that the defect will functionally close over time without the need for surgical repair [3] and development of heart failure. 25% of adults with small non-operated VSD demonstrated serious complications [4]. Few studies have investigated RV remodeling in rVSD due to the lack of overt symptoms since it is assumed that cardiac function reflects cellular/molecular function. It is unclear if subclinical remodeling is occurring as a prelude to impending dysfunction, therefore a biomarker and/or clinical test to predict RV dysfunction would enhance clinical care of rVSD patients.
Using a porcine model of rVSD, we tested the hypothesis that rVSD causes molecular remodeling and functional abnormalities of the RV. Detecting the presence of molecular remodeling may yield insights into novel targets for therapeutic intervention and lead to a fundamental change in the clinical management of rVSD by providing evidence for early repair of the defect.
2. Materials and methods
This study conformed to the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996). Domestic pigs (30–45 kg) underwent tiletamine–zolazepam sedation (6 mg/kg) and intubation. Ventilation maintained a PaCO2 of 40 mm Hg under 1.5–2.5% isoflurane anesthesia. Electrocardiography was performed for continuous lead II monitoring. Lactated Ringer's was infused throughout the procedure. Cefazolin (1 g) was administered for antibiotic prophylaxis.
2.1. Echocardiographic assessment of cardiac function
Cardiac function was assessed via transthoracic echocardiography (GE Healthcare Vivid 7; Andover, MA) at baseline, serially post-rVSD creation (weekly), and at study termination. Images were obtained at end-exhalation for the measurement of cardiac dimensions, LV ejection fraction (EF), and RV fractional area change, which has a high correlation to magnetic resonance imaging-obtained RV EF [5]. Color-flow Doppler was used to confirm rVSD creation and serially document rVSD patency. Pulsed-wave Doppler was used to assess mitral valve and tricuspid valve diastolic flow velocities for the measurement of early diastolic inflow velocity (E), late diastolic inflow velocity (A), and calculation of the E/A wave ratio. Parameters were averaged from multiple cardiac cycles.
2.2. rVSD creation
Muscular rVSD was created as previously described [6]. Briefly, a left mini-thoracotomy was performed to access the heart. Pledgeted polypropylene pursestring suture was placed in the LV lateral wall between the left anterior descending coronary artery and the circumflex coronary artery (avoiding major coronary branches). Heparin (100 U/kg IV) was administered. The VSD coring device (8 mm diameter) [6] was centered within the pursestring and quickly advanced through the LV lateral wall and across the ventricular septum under echocardiographic guidance. The coring device was withdrawn and the pursestring suture closed. Color-flow Doppler echocardiography was used to confirm rVSD creation. Arterial, mixed-venous, and venous blood samples were analyzed (i-STAT, Abbott Point-of-Care, Princeton, NJ) for the measurement of oxygen tension and pulmonary-to-systemic shunt (Qp:Qs ratio). Animals were weaned from ventilatory support, extubated, and returned to the animal vivarium. Bupivacaine (0.05–0.1 mg/kg) and buprenorphine (0.05 mg/kg) were administered as needed for analgesia. rVSD animals underwent weekly echocardiographic assessment of cardiac function and VSD patency under tiletamine–zolazepam sedation until the TV E/A wave ratio measured ≤1.0 [7]. Once this criterion was met, the terminal study was performed the following week.
For the terminal experiment, rVSD animals were anesthetized and echocardiographic data collection was repeated. Heparin was administered (100 U/kg) and introducer sheaths were placed into the carotid artery and external jugular vein. A pulmonary artery thermodilution catheter (7.5 Fr, Edwards Lifesciences; Irvine, CA) was passed into the RV and pulmonary artery for obtaining right heart hemodynamics and cardiac outputs. A dual-pressure micromanometer-tipped conductance catheter (Millar Instruments; Houston, TX) was passed into the aorta and LV for pressure–volume measurements [8,9]. Blood resistivity was measured to determine volume conductance. 10% hypertonic saline was bolused into the right heart to obtain parallel volume. An 8 Fr/22 Fr balloon occlusion catheter (Edwards) was rapidly inflated in the inferior vena cava for the measurement of load-independent hemodynamics. Data was measured at end-exhalation and collected across 10–15 cardiac cycles, acquired using a MPVS Ultra signal conditioner (Millar) with Power- Lab acquisition system (ADInstruments, Inc., Colorado Springs, CO), and analyzed using LabChart 5 Pro (ADInstruments) and PVAN Ultra (Millar) software. A median sternotomy was performed and the heart was excised. The right coronary artery was perfused with cold cardioplegia. The RV was excised for isolated myocyte experiments. Sections of LV and RV were formalin-fixed for immunohistochemistry and snap-frozen for Western blotting.
2.3. Isolation of ventricular myocytes
To determine whether rVSD is associated with impaired myocyte function, isolated myocyte experiments were performed using control and rVSD RV myocardium as described [10]. Immediately following heart harvest, the right coronary artery was cannulated and a piece of the RV was perfused with 110 ml of buffer solution (Ca2+ free, 37 °C, pH = 7.55 containing the following: 3 mM NTA, 100 g BSA (96%), and 6 mM ATP followed by Type II collagenase (0.5 mg/ml) into the buffer. Following 5 min of perfusion, 0.2 ml of 0.1 M CaCl2 solution was added and re-circulated (6 min). 0.2 ml of 0.1 M CaCl2 solution was then added and circulated (6 min). Solution A (pH 7.4) contained the following in mM: 38 K2HPO4, 79 KCl, 10 MgSO4, 5 HEPES, 20 taurine, 10 glucose, 5 creatine, and 8.3 succinate [11].
CaCl2 (0.2 ml of 0.1 M) was added to the perfusate and recirculated for 6 min for two cycles. Digested RV myocardium was minced in trituration solution (isolation buffer containing 0.5 mg/ml Type II collagenase, pH = 7.4), plus: 2.5 g BSA (96%), 50 units/ml DNase 1, 0.365 ml of 0.1 M CaCl2 solution, and 5 mM ATP [11]. Pieces were gently agitated, triturated, and oxygenated for 6 min. The solution–tissue mixture was filtered (100 µm screen) and centrifuged (520 rpm, 90 s). The pellet was resuspended in buffer (1× Media 199, pH = 7.4) containing 51 units/ml DNase 1 and 3.0 g/100 ml BSA and triturated until homogenous. Homogenized cells were incubated at 37 °C with 5% CO2 injection. Cell viability was ~80% as evidenced by rod-shaped myocytes. All experiments were performed within 8 h of isolation. Only rod-shaped myocytes with distinct sarcomeres and clear edges were selected for the recording of mechanical properties.
2.4. Cell shortening/relengthening measurements
Isolated myocyte contractile studies were performed as described [12,13] using a SoftEdge MyoCam® system (IonOptix Corporation, Milton, MA) and visualized with an inverted microscope. Cells were field stimulated with a suprathreshold voltage (1.0 Hz at 3 ms). All parameters were measured in at least 4–15 myocytes/heart.
2.5. SDS-PAGE/Western blot and protein identification
The intermediate filament protein desmin was used as a marker of RV cytoskeletal remodeling. SDS-PAGE and Western blot were performed on snap-frozen RV myocardium from both controls and rVSD animals using desmin antisera (#M0760, Dako, Glostrup, Denmark) as described [14]. Desmin immunohistochemistry was additionally performed on formalin-fixed RV control and rVSD myocardium using desmin antisera (1:150 dilution, #M0760, Dako) as described [12]. Images were acquired using an AxioSkop light microscope (Zeiss) with Olympus Magnafire camera under identical lighting conditions and optical settings. During the SDS-PAGE experiments, we observed several protein bands in Coomassie-stained gels from RV VSD myocardium which were increased in intensity compared to controls. Capillary-liquid chromatography–nanospray tandem mass spectrometry (Nano-LC/MS/MS) was used to identify these proteins [14].
2.6. Statistical analyses
Statistical analyses were performed using GraphPad Prism (GraphPad Software, Inc., San Diego, CA). Where appropriate, data are presented as mean ± SEM. p < 0.05 was considered significant. Following normality testing, data were analyzed using an unpaired t test or analysis of variance (ANOVA). Regression analysis was used to assess the inter-relationships between parameters.
3. Results
3.1. Creation of the animal model
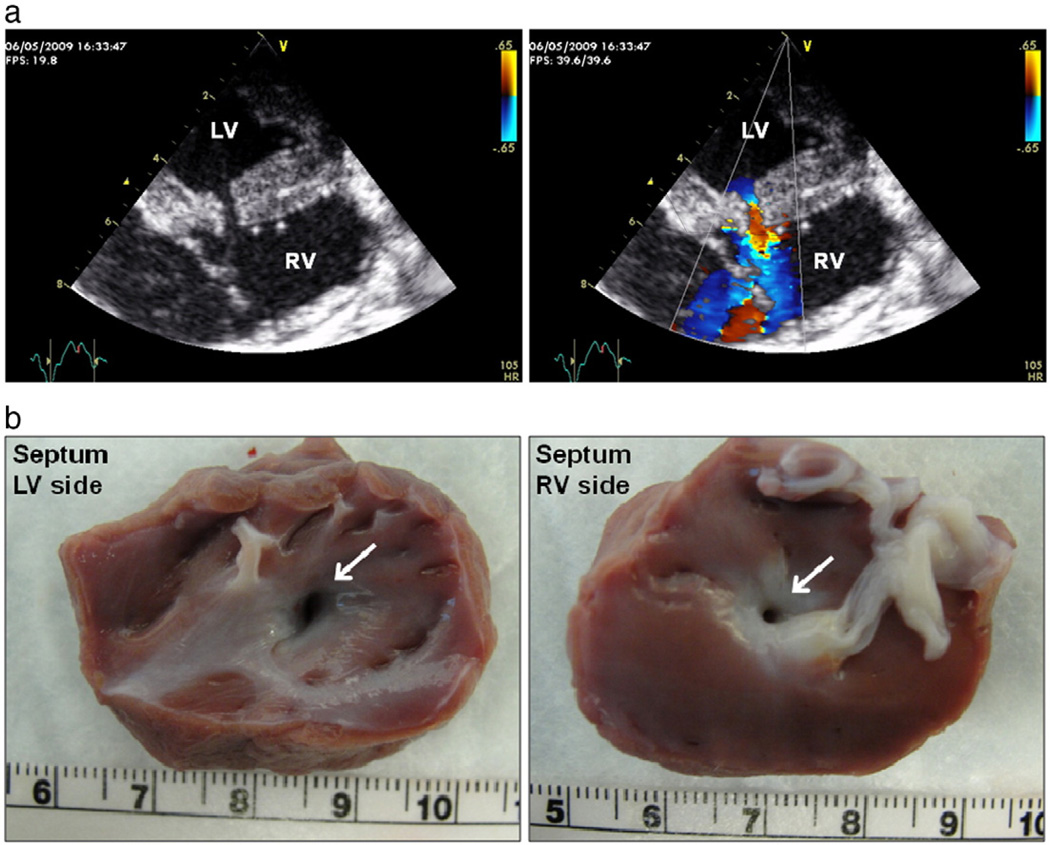
Five pigs weighing 30–45 kg underwent successful surgical creation of rVSD (Fig. 1a) and were carried out 1–6 weeks. Shunt fraction (Qp:Qs ratio) averaged 1.7 in the rVSD animals. Six pigs underwent acute control experiments (echocardiography, hemodynamics, and tissue harvest). rVSD patency was documented by colorflow Doppler echocardiography following rVSD creation, weekly, and at the terminal experiment (Fig. 1b). We used pigs weighing 30–45 kg in order to use our 6–8 Fr data acquisition catheters and ensure that the VSD was restrictive throughout the study. Smaller animals might better represent the neonate/pediatric population, although larger animals more faithfully represent the non-operated adult with rVSD.
Fig. 1.
rVSD creation. (a), Two-dimensional transepicardial echocardiography (left) and color-flow Doppler imaging (right) of a rVSD. (b), Patent rVSD (white arrows) from the LV (left) and RV (right) sides of the septum of an explanted rVSD heart.
3.2. Hemodynamic assessment of LV and RV functions in rVSD
The majority of RV hemodynamics was unchanged in rVSD compared to controls (Table 1). RV dP/dt and −dP/dt were decreased in rVSD and trended towards significance (p = 0.08 and p = 0.15, respectively). The RV contractility index (1/s), which is derived from dP/dt, was decreased 69% in rVSD (p = 0.02). These changes may reflect the subclinical onset of systolic and diastolic dysfunction. It is worth noting that these parameters were measured via intraventricular hemodynamic catheters which are not typically utilized in the clinical setting. Pulmonary artery diastolic pressure trended towards an increase in rVSD animals, measuring 3.2 ± 1.6 in controls and 8.3 ± 1.5 in rVSD (p = 0.08), although both values were within normal range. A potential limitation to our study is that the rVSD was surgically created in healthy animals through a wound in the LV lateral wall. This creates a small area of infarction, therefore rVSD creation may impact LV function and manifest as RV remodeling. To rule out this possibility we evaluated load-independent variables of LV performance: LV end-systolic pressure–volume relationship (ESPVR) slope, preload recruitable stroke work slope, and maximal elastance slope. These parameters were unchanged in rVSD compared to controls (Table 1). Furthermore, there were no significant differences in aortic or LV hemodynamics in rVSD animals compared to controls (Table 1). These findings demonstrate that rVSD creation did not negatively impact LV function in rVSD animals.
Table 1.
Hemodynamic parameters measured in control and rVSD pigs. Hemodynamics were measured from the right and left sides of the heart via a pulmonary artery thermodilution catheter and conductance catheter, respectively. Data are presented as mean ± SEM, n = 3–5 animals/group. There were no significant differences in any of these parameters between groups.
| Parameters | Control | rSVD |
|---|---|---|
| CVP Mean (mm Hg) | 1.17 ± 0.4 | 2.69 ± 0.6 |
| PAP Mean (mm Hg) | 8.05 ± 1.0 | 10.73 ± 1.3 |
| PAP Sys (mm Hg) | 11.74 ± 0.9 | 13.45 ± 1.1 |
| PAP Dia (mm Hg) | 3.15 ± 1.6 | 8.32 ± 1.5 |
| RV EDP (mm Hg) | 2.91 ± 0.2 | 3.27 ± 0.6 |
| RV Duration Sys (s) | 0.38 ± 0.04 | 0.37 ± 0.02 |
| RV Duration Dia (s) | 0.33 ± 0.01 | 0.23 ± 0.04 |
| RV dP/dt (mm Hg/s) | 271.80 ± 67.2 | 117.70 ± 6.2 |
| RV –dP/dt (mm Hg/s) | −203.40 ± 30.0 | −132.40 ± 26.8 |
| RV PTI (mm Hg s) | 3.78 ± 0.3 | 4.24 ± 0.6 |
| AoP Mean (mm Hg) | 60.39 ± 3.5 | 67.89 ± 8.0 |
| LVPmin (mm Hg) | 4.07 ± 3.1 | 3.41 ± 2.2 |
| LV EDP (mmHg) | 11.55 ± 1.5 | 15.99 ± 5.4 |
| LV Duration Sys (s) | 0.32 ± 0.02 | 0.33 ± 0.009 |
| LV Duration Dia (s) | 0.36 ± 0.05 | 0.26 ± 0.02 |
| LV dP/dt (mm Hg/s) | 1207.00 ± 129.2 | 1470.00 ± 98.7 |
| LV –dP/dt (mmHg/s) | −2174.00 ± 467.2 | −2088.00 ± 213.2 |
| LV CI (1/s) | 31.03 ± 0.2 | 31.31 ± 2.7 |
| LV Tau (s) | 0.026 ± 0.007 | 0.031 ± 0.002 |
| LV PTI (mm Hg*s) | 21.43 ± 3.0 | 25.63 ± 1.8 |
| CO/wt ((L/min)/kg) | 0.12 ± 0.01 | 0.15 ± 0.01 |
| ESPVR (slope) | 1.48 ± 0.3 | 1.66 ± 0.5 |
| PRSW (slope) | 47.33 ± 12.0 | 57.61 ± 11.2 |
| Emax (slope) | 3.71 ± 1.2 | 3.79 ± 0.8 |
Abbreviations: CVP Mean (mean central venous pressure); PAP Mean (mean pulmonary pressure); PAP Sys (pulmonary artery systolic blood pressure); PAP Dia (pulmonary artery diastolic blood pressure); EDP (enddiastolic pressure); Duration Sys (systolic duration); Duration Dia (diastolic duration); PTI (pressure–time index); AoP Mean (mean aortic blood pressure); LVPmin (LV minimum pressure); CI (contractility index); CO/wt (cardiac output normalized to body weight in kg); ESPVR (slope of the LV end-systolic pressure–volume relationship); PRSW (LV preload recruitable stroke work slope); Emax (LV maximal elastance slope).
3.3. Echocardiographic assessment of RV diastolic dysfunction in rVSD
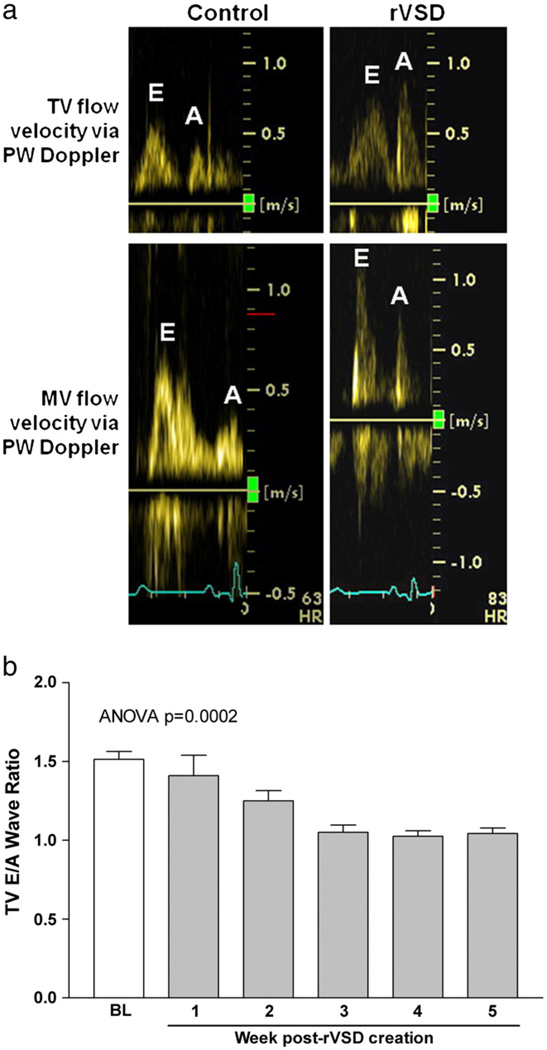
Color-flow Doppler confirmed successful rVSD creation intraoperatively and documented rVSD patency for the duration of the study (Fig. 1A). RV fractional area change, which correlates to magnetic resonance imaging-obtained RVEF [5], decreased 11% in rVSD compared to controls, although this difference was not statistically significant. RV free wall thickness was unchanged in rVSD (0.14 ± 0.03 mm/kg) versus controls (0.16 ± 0.02 mm/kg). Tricuspid valve diastolic in flow is used as a clinical parameter to assess diastolic function. To test the hypothesis that rVSD results in RV diastolic dysfunction we used pulsed-wave Doppler echocardiography (Fig. 2A). A tricuspid valve E/A wave ratio ≤1 was used as a criterion of RV diastolic dysfunction [7] and to determine study termination. The tricuspid valve E/A wave ratio measured 1.6 ± 0.05 in controls and 1.0 ± 0.08 in rVSD animals (p = 0.001). Serial measurements demonstrate the tricuspid valve E/A wave ratio progressively decreased in rVSD during the study (r2 = 0.88, p = 0.006, Fig. 2B). The tricuspid valve E/A wave ratio correlated to RV contractility index in both control and rVSD animals (r2 = 0.62, p = 0.06), indicating that the worse the animal's tricuspid valve E/A wave ratio, the lower the RV contractility index. The tricuspid valve E wave velocity measured 0.66 ± 0.06 in controls and 0.60 ± 0.04 in rVSD (p = ns). The tricuspid valve A wave velocity increased in rVSD, measuring 0.43 ± 0.03 in controls and 0.58 ± 0.08 in rVSD (p = 0.04). Serial measurements obtained within rVSD animals show the tricuspid valve A wave velocity progressively increased by 41% over time (r2 = 0.56, p = 0.05).
Fig. 2.
Echocardiographic assessment of RV diastolic dysfunction in rVSD. (a), Pulsed-wave Doppler echocardiography from a control and 6-week rVSD animal demonstrates inversion of the tricuspid valve early (E) and late (A)diastolic in flow velocity ratio (E/Aratio) in rVSD. A tricuspid valve E/A ratio < 1.0 was used to define RV diastolic dysfunction (and study termination). The mitral valve E/A ratio remained unchanged in rVSD compared to controls. (b), Progressive decline in the tricuspid valve E/A ratio over time in rVSD animals (n = 3). The baseline column is combined from controls (n = 5) and baseline rVSD (n = 3) data.
One possible explanation for the data is that there is diastolic dysfunction of the entire heart. To rule out this possibility, we tested whether the LV demonstrated evidence of diastolic dysfunction. Pulsed-wave Doppler examination of mitral valve diastolic flow pattern showed no differences in the mitral valve E/A ratio in rVSD pigs compared to controls (Fig. 2). The mitral valve E/A ratio measured 1.6 ± 0.07 in controls and 1.5 ± 0.01 in rVSD. There were no significant differences in LVEF or LV chamber areas in rVSD compared to controls. LVEF measured 49.4 ± 1.8% in controls and 43.3 ± 3.3% in rVSD. LV short-axis end-systolic area (LVESA) measured 0.19 ± 0.03 cm2/kg in controls and 0.18 ± 0.03 cm2/kg in rVSD. LV short-axis end-diastolic area (LVEDA) measured 0.38 ± 0.05 cm2/kg in controls and 0.31 ± 0.03 cm2/kg in rVSD. These findings demonstrate that surgical rVSD creation did not negatively impact LV function.
3.4. Molecular remodeling in rVSD RV myocardium
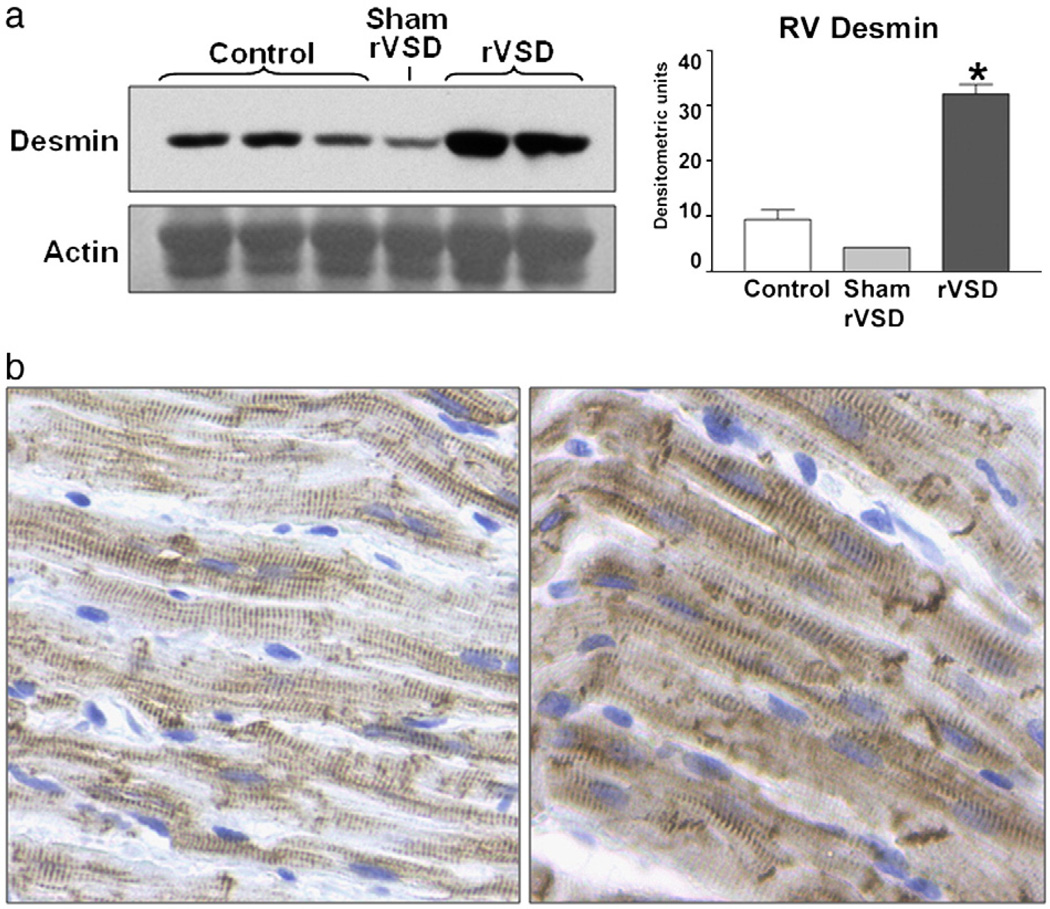
It is expected that molecular changes precede functional changes during the remodeling process. To determine if RV myocardium undergoes molecular remodeling in rVSD, we performed Western blot analysis to probe for the cytoskeletal intermediate filament protein desmin. Desmin upregulation is noted to occur in the LV and is a robust marker for myocardial remodeling [14]. Strikingly, despite the lack of overt RV hemodynamic abnormalities, desmin protein content was significantly increased in rVSD RV myocardium (Fig. 3a). Densitometric analysis of desmin Western blot band intensity revealed that desmin protein content increased 285% in rVSD RV myocardium compared to controls (p = 0.0011). To qualitatively assess desmin content in RV myocytes, we performed desmin immunohistochemistry on control and rVSD myocardium (Fig. 3b). rVSD RVmyocytes demonstrated increased desmin stain intensity compared to control images, indicating desmin upregulation. Desmin stain intensity was uniform throughout rVSD myocytes and prominent at intercalated discs. rVSD myocytes were slightly larger in size compared to controls, possibly indicating the onset of myocyte hypertrophy.
Fig. 3.
Desmin protein upregulation in rVSD RV myocardium. (a), Western blot of RV desmin protein from control, sham-operated, and rVSD animals (40 µg protein/lane) demonstrating significant desmin upregulation in rVSD RV myocardium. Data is presented as mean ± SEM. Data was analyzed using a two-tailed unpaired t test with p < 0.05 considered significant. (b), desmin immunohistochemistry performed on control (left) and 6-week rVSD (right) RV myocardium (images at 40×). Note the increased desmin stain intensity in rVSD myocytes, both uniformly throughout the cell and at the intercalated discs.
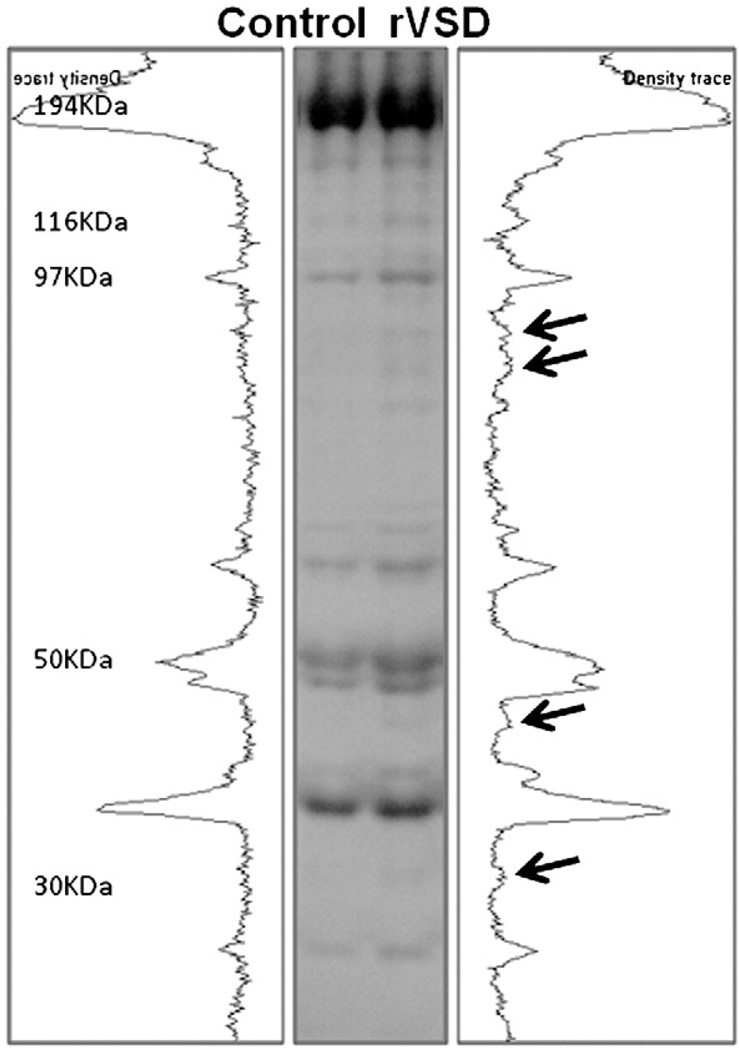
During SDS-PAGE experiments, we observed multiple protein bands in Coomassie-stained gels which were markedly increased in intensity in rVSD RV myocardium compared to controls (Fig. 4). The protein bands were excised from the gel and identified using proteomics techniques. Nano-LC/MS/MS was used to identify four of these upregulated proteins as mitochondrial energetics proteins involved in the citric acid cycle and beta-oxidation pathway. Aconitate hydratase (ACO2), which catalyzes citrate to isocitrate in the citric acid cycle, was increased in our rVSD model and is upregulated in human VSD myocardium [2,15]. Mitochondrial aspartate aminotransferase (GOT2), another citric acid cycle isoenzyme that interconverts aspartate and oxaloacetate, was increased in rVSD [16]. rVSD RV myocardium demonstrated increases in both trifunctional enzyme subunit α and hydroxyacyl-coenzyme A dehydrogenase. The trifunctional enzyme is involved in three steps of the beta-oxidation of fatty acids [17]. The α subunit (HADHA) contains 2-enoyl-CoA hydratase and 3-hydroxyacyl- CoA dehydrogenase activities. As part of the trifunctional enzyme, hydroxyacyl-CoA dehydrogenase catalyzes oxidation of straight-chain 3-hydroxyacyl-CoAs in beta-oxidation [18]. Increased beta-oxidation proteins suggest that rVSD myocytes were in normoxic conditions [19].
Fig. 4.
Mitochondrial protein upregulation in rVSD RV myocardium. Coomassie gel containing representative control and rVSD protein lanes. Molecular weights (kilodaltons) are at left. Arrows point to the densitometric peaks of four upregulated proteins. These four protein bands of interest were excised and identified using proteomics techniques (nano-LC/MS/MS) as mitochondrial energetics proteins involved in the citric acid cycle and beta-oxidation pathway (Table 2).
3.5. Abnormal contraction and relaxation parameters in rVSD RV isolated myocytes
Although clinically applicable, cardiac function at the whole animal level could be confounded by other variables. To validate our echocardiographic and hemodynamic observations we performed experiments on isolated myocytes from control and rVSD animals to determine if contraction/relaxation abnormalities exist at the cellular level (Table 3). Consistent with diastolic dysfunction, rVSD RV myocytes demonstrated relaxation abnormalities with decreased magnitude and rate of response. Additionally, we observed abnormalities in isolated myocyte contraction, with decreased magnitude and rate of response in the rVSD RV myocytes compared to controls. The findings of RV relaxation abnormalities in rVSD RV myocytes may explain the diastolic dysfunction measured via echocardiography and may be in part due to increased desmin protein content in the myocytes (akin to intracellular remodeling).
Table 3.
Altered RV isolated myocyte contractile and relaxation in rVSD. Isolated myocyte functional studies were performed on RV myocytes from control and rVSD animals immediately following heart harvest to assess contraction/relaxation parameters. Data are presented as the mean ± SEM from 4 myocytes per animal (n = 5 control and n = 3 rVSD pigs). Data was analyzed using a two-tailed unpaired t test with p < 0.05 considered significant.
| Parameters | Control (n = 20 cells) | rVSD (n = 12 cells) | p |
|---|---|---|---|
| Baseline sarcomere length, µ | 2.68 ± 0.2 | 2.14 ± 0.03 | 0.05 |
| Contraction parameters | |||
| Departure velocity, mm/s | −2.50 ± 0.33 | −1.23 ± 0.3 | 0.01 |
| Departure velocity time, s | 0.071 ± 0.007 | 0.11 ± 0.02 | 0.03 |
| Peak, mm | 2.41 ± 0.2 | 1.99 ± 0.02 | 0.09 |
| Peak height, mm | 0.26 ± 0.03 | 0.15 ± 0.03 | 0.01 |
| Baseline% peak height,% | 10.2 ± 0.9 | 6.98 ± 1.3 | 0.04 |
| Peak time, s | 0.33 ± 0.02 | 0.49 ± 0.03 | 0.0004 |
| Time to peak 50%, s | 0.10 ± 0.007 | 0.16 ± 0.03 | 0.008 |
| Time to peak 90%, s | 0.21 ± 0.01 | 0.33 ± 0.04 | 0.001 |
| Relaxation parameters | |||
| Return velocity, mm/s | 2.85 ± 0.42 | 1.44 ± 0.42 | 0.03 |
| Return velocity time, s | 0.45 ± 0.04 | 0.73 ± 0.06 | 0.0002 |
| Time to baseline 50%, s | 0.46 ± 0.04 | 0.73 ± 0.05 | 0.0001 |
| Time to baseline 90%, s | 0.55 ± 0.04 | 0.82 ± 0.06 | 0.0003 |
4. Discussion
Using a porcine model as a platform for our investigations, we are the first to report the presence of RV diastolic dysfunction, molecular alterations, and myocyte contractile/relaxation impairment in rVSD despite the absence of hemodynamic abnormalities. Our model is similar clinically to rVSD patients that have normal echocardiographic exam (RV systolic function), normal central venous pressure, and normal pulmonary artery pressure. One translational advantage of our study is that echocardiography, which is a utilitarian, non-invasive, clinical imaging modality, identified the onset of RV diastolic dysfunction in rVSD and therefore may have immediate clinical applications. A second clinically applicable outcome of our study is that desmin protein was increased in rVSD RV myocardium. Desmin is clinically utilized as an oncological marker for muscle-derived tumors and may represent a novel biomarker for impending RV dysfunction in rVSD. Importantly, detecting the presence of subclinical remodeling may alter the clinical management of rVSD by providing evidence for early repair of the defect.
The crux of the clinical management dilemma in patients with VSD is avoidance of pulmonary hypertension and RV failure. Large VSDs remain patent (nonrestrictive VSD), creating significant blood shunting (Qp/Qs ratio ≥ 2.0) and high ventricular pressure gradients. Increased pulmonary blood flow (Qp) via the shunt can result in pulmonary arterial reactivity and eventually pulmonary hypertension and RV failure. If left uncorrected, 20% of infants with large VSDs develop pulmonary vascular disease and 25% develop HF in the first year of life [3]. Pulmonary vascular resistance becomes fixed and surgical correction does not correct the physiological perturbations. Combined heart–lung transplantation is the only therapeutic option. Large VSDs are usually repaired within 12 months of age to prevent pulmonary hypertension [20].
rVSDs that do not result in high pressure gradients and clinically significant shunting (Qp/Qs ≤ 1.99) typically demonstrate little/no associated hemodynamic abnormalities as currently measured. Clinical management of rVSD is more complicated: rVSD is observed for symptoms of HF or pulmonary hypertension and continued observation for rVSD without corrective intervention [21] with the hope that the defect will functionally close (myocyte growth and/or systolic compression) over time. 25% of adults with small VSD demonstrated serious complications [4]. Controversy exists as to how the risk of surgical closure in an asymptomatic child weighs against the lifetime risk of an isolated, non-operated rVSD [22]. Without clearly defined evidence supporting optimal care, clinicians are unable to predict which rVSD patients have an increased risk of a poor outcome.
There is a paucity of literature investigating the molecular alterations in VSD and rVSD; we are the first to report molecular and functional changes in rVSD. Serial acquisition of myocardium in an asymptomatic rVSD patient is not feasible and there are few animal models to study this important problem. The majority of VSD animal model literature has focused on hemodynamics and/or device testing [23,24], although progress is being made in understanding the genetics behind VSD [2]. Desmin increases in the LV during ischemic HF and is a more robust predictor of cardiac dysfunction than fibrosis and myocyte hypertrophy [14]. Upregulation may acutely counter increased wall tension secondary to increase filling pressures, reinforce myocyte integrity, and stabilize sarcomeres [14,25]. Desmin upregulation also occurs in myocardium remote from dysfunctional regions [14], suggesting that the stimuli behind desmin upregulation is not exclusively ischemia but includes perturbations such as wall stretch/stress. Despite the absence of hemodynamic alterations and significant shunt, we demonstrate marked upregulation of the cytoskeletal protein desmin in rVSD RV myocardium. Desmin is an intermediate filament unique to muscle. In healthy myocardium, desmin surrounds the Z-discs of sarcomere myofilaments, forming a filamentous lattice to sustain alignment within myocytes [26]. Desmin supports myocyte architecture during cardiac contraction, reinforcing the cytoskeleton and preventing sarcomere slippage to enable force generation [27], and serves as a stress-bearing element in the sarcomere [25]. Cytoskeletal abnormalities, particularly desmin upregulation, may represent a common pathway for dysfunction in myocardium [26]. The presence of desmin upregulation in rVSD RV myocardium suggests that cytoskeletal remodeling is occurring. Desmin upregulation may decrease myocyte compliance, manifesting clinically as diastolic dysfunction. Our findings demonstrate that desmin upregulation is an early molecular adaptation in rVSD RV myocardium and precedes functional alterations (single myocyte and RV diastolic dysfunction). Although causality cannot be inferred, this would be a requisite observation if desmin upregulation is indeed a mechanism for the development of RV diastolic dysfunction.
Desmin accumulation may be deleterious to cardiac performance. Desmin is stiff and among the least soluble cellular components [14] and may contribute to RV diastolic dysfunction by increasing stiffness within myocytes. In our model, heightened energy demands by the RV may be crucial to overcome the presence of RV diastolic dysfunction, myocyte contractile derangement, and upregulation of desmin in rVSD. Although dissecting mechanisms resulting in desmin upregulation in rVSD is beyond the scope of this study, further research is warranted to determine if increased desmin is the cause of or a reaction to RV diastolic dysfunction. RV myocardial hypertrophy (wall thickness), pressure-overload secondary to pulmonary hypertension, or ischemia is not responsible for diastolic dysfunction in our model.
Abnormalities at the cellular level, however, could potentially explain the diastolic dysfunction. We investigated this possibility and discovered that rVSD resulted in both contraction and relaxation abnormalities in RV myocytes. RV myocytes demonstrated evidence of relaxation abnormalities in rVSD as shown by a shortened return velocity with a prolonged return velocity time (Table 3). This correlates with our hemodynamic finding of RV −dP/dt and our echocardiographic finding of abnormal TV diastolic inflow. Hemodynamically, RV dP/dt and −dP/dt appear to be diminished in rVSD although the changes did not achieve statistical significance. If indeed they are depressed, this would provide further evidence for systolic and diastolic RV dysfunction at the level of the heart. These alterations appeared to be limited to the RV only since LV dP/dt and −dP/dt were unchanged. These observations demonstrate that rVSD results in RV diastolic dysfunction. Unexpectedly, there appeared to be evidence of systolic dysfunction, although it was not as prominent as diastolic dysfunction. Despite normal systolic findings on echocardiography, the RV demonstrated decreased dP/dt and impaired isolated myocyte contraction. Myocyte dysfunction could be due to abnormal signal transduction, myocyte compliance, and/or energy metabolism. The myocardium maintains considerable energy demands in healthy systems. Indices such as intramyocardial pressure and tension-time index are costly in terms of energy requirements and are well-characterized in the LV [9], however the energy requirements of the RV, especially in rVSD, are unclear. Increases in mitochondrial energetic proteins, either via increased protein upregulation or by increased mitochondrial biosynthesis, may reflect a boost in energy production essential for fulfilling enhanced demands by the RV [2,28]. We are currently investigating these potential mechanisms.
In summary, few studies have investigated the potential for RV remodeling since it is assumed that whole heart function is a reflection of conditions at the cellular and molecular levels. Our model of rVSD demonstrated molecular remodeling and progressive RV diastolic dysfunction. The RV myocardium undergoes subclinical remodeling encompassing diastolic dysfunction, impairment of both contraction and relaxation function in isolated RV myocytes, upregulation of mitochondrial energetics proteins, and upregulation of the cytoskeletal protein desmin. Desmin may represent a key subclinical early marker for future RV dysfunction. In our model, rVSD appears to only affect the RV and not the LV. This is not surprising: rVSD is a left-to-right shunt and preferentially exposes the RV to mechanical forces or increased volume. Because the RV is a low-pressure system, small changes in RV compliance may have significant clinical consequences. Evaluating tricuspid valve diastolic inflow patterns may be a useful assessment for stratifying risk in rVSD patients. An improved understanding of RV dysfunction and/or the mechanisms resulting in RV remodeling may have clinical applications extending beyond rVSD interventions. While RV failure is the primary medical issue in essentially all pediatric heart defects, diastolic dysfunction and RV failure are also prominent concerns in ischemic HF, valvular heart disease, pulmonary hypertension, and heart/lung transplantation. A major translational outcome of this project is that patients with rVSD should be carefully examined for RV diastolic dysfunction. Repairing the rVSD may attenuate or reverse RV remodeling and prevent the disease from progressing into pulmonary hypertension and HF. Collectively these findings may lead to a fundamental change in the clinical management of rVSD by providing evidence for early repair of the defect.
Table 2.
Mitochondrial protein upregulation in rVSD RV myocardium. Results of protein identification of four unknown Coomassie-stained SDS-PAGE bands (Nano-LC/MS/MS) upregulated in rVSD RV myocardium.
| Protein | MudPIT score | Mass (KDa) | pI | % Sequence match | %Δ in rVSD |
|---|---|---|---|---|---|
| ACO2 | 1716 | 86.5 | 8.24 | 44% | +77%* |
| HADHA | 1745 | 83.7 | 9.28 | 45% | +91%* |
| GOT2 | 1411 | 47.8 | 9.14 | 54% | +78%* |
| HADH | 734 | 34.2 | 9.02 | 50% | +56%* |
pI, isoelectric point; KDa, protein mass (kilodaltons). ACO2, aconitate hydratase; HADHA, trifunctional enzyme subunit α; GOT2, aspartate aminotransferase; HADH, hydroxyacyl-coA dehydrogenase. Data was analyzed using a two-tailed unpaired t test.
p < 0.05 considered significant.
Acknowledgments
Disclosures
Funding was provided by the Society of Cardiovascular Anesthesiologists MidCareer Grant and the Heart Center Translational Research Grant from Nationwide Children's Hospital, Columbus, Ohio.
The authors thank ULAR Surgery for their assistance, Kari Green- Church, PhD and Cindy James, PhD for their help with proteomic experiments, and the Department of Surgery at The Ohio State University for their support.
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. AHA statistical update. Heart disease and stroke statistics — 2007 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Zhang H, Zhou L, Yang R, Sheng Y, Sun W, Kong X, et al. Identification of differentially expressed genes in human heart with ventricular septal defect using suppression subtractive hybridization. Biochem Biophys Res Commun. 2006;342:135–144. doi: 10.1016/j.bbrc.2006.01.113. [DOI] [PubMed] [Google Scholar]
- 3.Demirag MK, Keçeligil HT, Kolbakir F. Primary surgical repair of ventricular septal defect. Asian Cardiovasc Thorac Ann. 2003;11:213–216. doi: 10.1177/021849230301100307. [DOI] [PubMed] [Google Scholar]
- 4.Neumayer U, Stone S, Somerville J. Small ventricular septal defects in adults. Eur Heart J. 1998;19:1573–1582. doi: 10.1053/euhj.1998.1083. [DOI] [PubMed] [Google Scholar]
- 5.Horton KD, Meece RW, Hill JC. Assessment of the right ventricle by echocardiography: a primer for cardiac sonographers. J Am Soc Echo. 2009;22:776–792. doi: 10.1016/j.echo.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 6.Phillips AB, Green J, Bergdall VK, Yu J, Monreal G, Gerhardt MA, et al. Teaching the “hybrid approach”: a novel swine model of muscular ventricular septal defect. Ped Card. 2009;30:114–118. doi: 10.1007/s00246-008-9297-x. [DOI] [PubMed] [Google Scholar]
- 7.Oh JK, Seward JB, Tajik JA. The echo manual. second edition. Philadelphia, Pennsylvania: Lippincott Williams & Wilkins; 1999. [Google Scholar]
- 8.Monreal G, Gerhardt MA, Kambara A, Abrishamchian AR, Bauer JA, Goldstein AH. Selective microembolization of the circumflex coronary artery in an ovine model: dilated, ischemic cardiomyopathy and left ventricular dysfunction. J Card Fail. 2004;10:174–183. doi: 10.1016/j.cardfail.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein AH, Monreal G, Kambara A, Spiwak AJ, Schlossberg ML, Abrishamchian AR, et al. Partial support with a centrifugal left ventricular assist device reduces myocardial oxygen consumption in chronic, ischemic heart failure. J Card Fail. 2005;11:142–151. doi: 10.1016/j.cardfail.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Wold LE, Ceylan-Isik AF, Fang CX, Yang X, Li SY, Sreejayan N, et al. Metallothionein alleviates cardiac dysfunction in streptozotocin-induced diabetes: role of Ca2+ cycling proteins, NADPH oxidase, poly(ADP-Ribose) polymerase and myosin heavy chain isozyme. Free Radic Biol Med. 2006;40:1419–1429. doi: 10.1016/j.freeradbiomed.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Zellner JL, Spinale FG, Eble DM, Hewett KW, Crawford FA., Jr Alterations in myocyte shape and basement membrane attachment with tachycardia-induced heart failure. Circ Res. 1991;69:590–600. doi: 10.1161/01.res.69.3.590. [DOI] [PubMed] [Google Scholar]
- 12.Ren J, Wold LE. Measurement of cardiac mechanical function in isolated ventricular myocytes from rats and mice by computerized video-based imaging. Biol Proced Online. 2001;3:43–53. doi: 10.1251/bpo22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wold LE, Saari JT, Ren J. Isolated ventricular myocytes from copper-deficient rat hearts exhibit enhanced contractile function. Am J Physiol Heart Circ Physiol. 2001;281:H476–H481. doi: 10.1152/ajpheart.2001.281.2.H476. [DOI] [PubMed] [Google Scholar]
- 14.Monreal G, Nicholson LM, Han B, Joshi MS, Phillips AB, Wold LE, et al. Cytoskeletal remodeling of desmin is a more accurate measure of cardiac dysfunction than fibrosis or myocyte hypertrophy. Life Sci. 2008;83:786–794. doi: 10.1016/j.lfs.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 15.Matasova LV, Popova TN. Aconitate hydratase of mammals under oxidative stress. Biochemistry (Mosc) 2008;73:957–964. doi: 10.1134/S0006297908090010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doonan S, Martini F, Angelaccio S, Pascarella S, Barra D, Bossa F. The complete amino acid sequences of cytosolic and mitochondrial aspartate aminotransferases from horse heart, and inferences on evolution of the isoenzymes. J Mol Evol. 1986;23:328–335. doi: 10.1007/BF02100642. [DOI] [PubMed] [Google Scholar]
- 17.Eaton S, Bursby T, Middleton B, Pourfarzam M, Mills K, Johnson AW, et al. The mitochondrial trifunctional protein: centre of a beta-oxidation metabolon? Biochem Soc Trans. 2000;28:177–182. doi: 10.1042/bst0280177. [DOI] [PubMed] [Google Scholar]
- 18.Yang SY, He XY, Schulz H. 3-Hydroxyacyl-CoA dehydrogenase and short chain 3-hydroxyacyl-CoA dehydrogenase in human health and disease. FEBS J. 2005;272:4874–4883. doi: 10.1111/j.1742-4658.2005.04911.x. [DOI] [PubMed] [Google Scholar]
- 19.McArdle WD, Katch FI, Katch VL. Exercise physiology. Philadelphia, Pennsylvania: Lippincott, Williams, & Wilkins; 2007. [Google Scholar]
- 20.Yacoub MH, Radley-Smith R, De Gasperis C. Primary repair of large ventricular septal defect in the first year of life. G Ital Cardiol. 1978;8:827–831. [PubMed] [Google Scholar]
- 21.Nygren A, Sunnegårdh J, Berggren H. Preoperative evaluation and surgery in isolated ventricular septal defects: a 21 year perspective. Heart. 2000;83:198–204. doi: 10.1136/heart.83.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Backer CL, Winters RC, Zales VR, Takami H, Muster AJ, Benson DW, Jr, et al. Restrictive ventricular septal defect: how small is too small to close? Ann Thorac Surg. 1993;56:1014–1019. doi: 10.1016/0003-4975(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 23.Corin WJ, Swindle MM, Spann JF, Jr, Nakano K, Frankis M, Biederman RW, et al. Mechanism of decreased forward stroke volume in children and swine with ventricular septal defect and failure to thrive. J Clin Invest. 1988;82:544–551. doi: 10.1172/JCI113630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding ZR, Qin YW, Hu JQ, Zhao XX, Yang ZH, Hong-Wu, et al. A new pan-nitinol occluder for transcatheter closure of ventricular septal defects in a canine model. J Interv Cardiol. 2009;22:191–198. doi: 10.1111/j.1540-8183.2009.00432.x. [DOI] [PubMed] [Google Scholar]
- 25.Di Somma S, Marotta M, Salvatore G, Cudemo G, Cuda G, De Vivo F, et al. Changes in myocardial cytoskeletal intermediate filaments and myocyte contractile dysfunction in dilated cardiomyopathy: an in vivo study in humans. Heart. 2000;84:659–667. doi: 10.1136/heart.84.6.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capetanaki Y. Desmin cytoskeleton in healthy and failing heart. Heart Fail Rev. 2000;5:203–220. doi: 10.1023/A:1009853302447. [DOI] [PubMed] [Google Scholar]
- 27.Boriek AM, Capetanaki Y, Hwang W, Officer T, Badshah M, Rodarte J, et al. Desmin integrates the three-dimensional mechanical properties of muscles. Am J Physiol. 2001;280:C46–C52. doi: 10.1152/ajpcell.2001.280.1.C46. [DOI] [PubMed] [Google Scholar]
- 28.Rimbaud S, Garnier A, Ventura-Clapier R. Mitochondrial biogenesis in cardiac pathophysiology. Pharmacol Rep. 2009;61:131–138. doi: 10.1016/s1734-1140(09)70015-5. [DOI] [PubMed] [Google Scholar]