Multivariate survival analyses, visual observations, and receiver operating characteristic comparisons helped demonstrate that, compared with conventional segmentation, contrast-enhanced T1-weighted subtraction maps of contrast-enhancing lesions produce better stratification of high- and low-risk patients treated with bevacizumab and therefore should be used in future clinical trials involving evaluation of antiangiogenic therapies in brain tumors.
Abstract
Purpose
To compare the capability to aid prediction of clinical outcome measures, including progression-free survival (PFS) and overall survival (OS), between volumetric estimates from contrast material–enhanced (CE) T1-weighted subtraction maps and traditional segmentation in a randomized multicenter clinical trial of recurrent glioblastoma (GBM) patients treated with bevacizumab.
Materials and Methods
All patients participating in this study signed institutional review board–approved informed consent at their respective institutions prior to enrolling in the multicenter clinical trial. One-hundred sixty patients with recurrent GBM enrolled as part of a HIPAA-compliant, multicenter clinical trial (AVF3708 g, BRAIN trial). Contrast-enhancing tumor volumes and change in volumes as a response to therapy were quantified by using either conventional segmentation or CE T1-weighted subtraction maps created by voxel-by-voxel subtraction of intensity-normalized nonenhanced T1-weighted images from CE T1-weighted images. These volumes were then tested as predictors of PFS and OS by using log-rank univariate analysis, the multivariate Cox proportional hazards regression model, and receiver operating characteristic analysis.
Results
Use of CE T1-weighted subtraction maps qualitatively improved visualization and improved quantification of tumor volume after bevacizumab treatment. Significant trends between the volume of tumor and change in tumor volume after therapy on CE T1-weighted subtraction maps were found for both PFS and OS (pretreatment volume < 15 cm3, P < .003; posttreatment volume < 7.5 cm3, P < .05; percentage change in volume > 25%, P = .004 for PFS and P = .053 for OS). CE T1-weighted subtraction maps were significantly better at aiding prediction of 6-month PFS and 12-month OS compared with conventional segmentation by using receiver operating characteristic analysis (P < .05).
Conclusion
Use of CE T1-weighted subtraction maps improved visualization and aided better prediction of patient survival in recurrent GBM treated with bevacizumab compared with conventional segmentation of CE T1-weighted images.
©RSNA, 2013
Clinical trial registration no. NCT00345163
Introduction
Since accelerated Food and Drug Administration approval in May 2009, the standard of care for recurrent glioblastoma (GBM) in the United States has become bimonthly treatment with bevacizumab (Avastin; Genentech, South San Francisco, Calif) at a dose of 10 mg per kilogram body weight, a humanized monoclonal antibody that inhibits vascular endothelial growth factor A, or VEGF-A, with or without a chemotherapeutic agent. Initial reports of bevacizumab treatment in recurrent GBM were remarkable (1–6), with almost complete loss of contrast enhancement in most of the patients. This degree of enthusiasm was muted, as reports began to describe startling recurrence patterns consistent with infiltrating and nonenhancing tumor (5–11). It appeared as though bevacizumab reduced the degree of contrast enhancement on contrast material–enhanced (CE) T1-weighted images independent of changes in tumor burden (12). This has led to the reassessment (13) of conventionally defined tumor response criteria (ie, the criteria of Macdonald et al [14]), which traditionally utilized bidimensional measurements of contrast-enhancing tumor on CE T1-weighted magnetic resonance (MR) images. Because bevacizumab plays an important role in treatment of recurrent GBM, there is increased urgency for developing imaging biomarkers to aid in the assessment of individual patient responses to bevacizumab therapy. In addition, an easily implementable screening biomarker for preselection of patients who will benefit most from bevacizumab could play an important role in cohort enrichment for the next phase of combination therapies.
To date, investigators in single-institution studies have identified only weak relationships among tumor volumes, change in tumor volumes in response to bevacizumab therapy, and patient survival in recurrent GBM treated with bevacizumab (15), probably because of the difficulty in defining subtle enhancing tumor boundaries after the start of therapy. We hypothesize that digital subtraction of nonenhanced T1-weighted images from CE T1-weighted images, or CE T1-weighted subtraction maps, a form of which was originally proposed by Bedekar et al (16,17), may improve visualization and quantification of subtly enhancing tumor in recurrent GBM treated with bevacizumab. Digital subtraction techniques are commonly used to increase contrast-to-noise ratios in applications requiring detection of subtle changes in contrast material uptake, including identification of vascular enhancement patterns by using digital subtraction angiography (18,19), or detection of subtly enhancing body tumors by using static (20) and dynamic CE subtraction MR imaging (21,22). In the current study, we compared the capability to aid prediction of clinical outcome measures (progression-free survival [PFS] and overall survival [OS]) between volumetric estimates from CE T1-weighted subtraction maps and traditional segmentation in a randomized multicenter clinical trial of recurrent GBM in patients treated with bevacizumab.
Materials and Methods
BRAIN Trial Study Patient Population
All patients participating in the BRAIN trial (study sponsor identification, AVF3708g; clinical trial registration no. NCT00345163) signed an institutional review board–approved informed consent at their respective institution prior to enrolling in the multicenter clinical trial. The current imaging analysis was performed retrospectively by using anonymized data from the study sponsor (Genentech). Three authors had control of both imaging data (B.M.E., T.F.C.) and clinical outcome data (H.J.K.). The BRAIN trial was an open-label, multicenter (11 sites), randomized, noncomparative phase II trial performed to assess the effectiveness of bevacizumab (dose, 10 mg/kg) or bevacizumab and irinotecan hydrochloride injection (Camptosar; Pfizer, New York, NY), also known as CPT-11, at a dose of 340 mg/m2 or 125 mg/m2 (with or without concomitant enzyme-inducing antiepileptic drugs) in patients with recurrent, histologically confirmed GBM (23). The study spanned from July 2006 through September 2007. Specific inclusion and exclusion criteria for this clinical trial can be found at clinicaltrials.gov/ct2/show/NCT00345163. All enrolled patients in whom both nonenhanced T1-weighted images and CE T1-weighted images were available were included. A total of 160 of 167 patients with recurrent GBM (one or two relapses) enrolled in this multicenter trial. In these patients, MR imaging data of sufficient quality (ie, no geometric distortions) and both nonenhanced T1-weighted images and CE T1-weighted images were available for subsequent CE T1-weighted subtraction map analysis. In the seven patients who were excluded, both the nonenhanced T1-weighted images and CE T1-weighted images necessary for generating CE T1-weighted subtraction maps were not available. In all patients, initial standard radiation therapy and chemotherapy (concurrent radiation therapy and temozolomide treatment) failed, and radiation therapy had been completed more than 8 weeks previously. Baseline images were obtained prior to treatment, and follow-up images were obtained at approximately 6 weeks after treatment, according to study guidelines. In the study population, the average age was 54.4 years (range, 23–79 years), and the mean Karnofsky Performance Score (scale of 0–100) was 81.8 ± 0.8 (standard error of the mean); 109 of 160 patients were male (68%) and 51 of 160 patients were female (32%). In the male patients, the average age was 55.6 years (range, 24–76 years) and the mean Karnofsky Performance Score was 81.9 ± 0.9. In the female patients, the average age of 52.3 years (range, 23–79 years), and the mean Karnofsky Performance Score was 81.6 ± 1.4. Eight of 160 patients (5%) had progression by the first follow-up and were excluded from posttreatment tumor volume measurement and subsequent analyses. A total of 130 of 160 patients (81%) were treated at the first recurrence, whereas 30 of 160 patients (19%) were treated at the second recurrence. At the time of last assessment (November 2012), 141 of 160 patients (88%) were deceased.
MR Images
Standard anatomic MR data were acquired by using either a 1.5-T or a 3-T MR imaging unit, manufactured by GE Medical Systems (Milwaukee, Wis), Siemens Healthcare (Erlangen, Germany), Philips Medical Systems (Best, the Netherlands), or Hitachi Medical Corporation (Tokyo, Japan), at 11 participating sites. Standard anatomic images were obtained with the axial T1-weighted fast spin-echo sequence or magnetization-prepared rapid acquisition gradient-echo sequence (repetition time msec/echo time msec/inversion time msec, 400–3209/3.6–21.9/0–1238; section thickness, 3–6.5 mm; intersection gap, 0–2.5 mm; number of signals acquired, one to two; matrix size, 176–512 × 256–512; and field of view, 24–25.6 cm), the T2-weighted fast spin-echo sequence, and the fluid-attenuated inversion-recovery sequence. In addition, axial T1-weighted images enhanced with gadopentetate dimeglumine (Magnevist; Berlex), 0.1 mmol/kg, and acquired shortly after contrast material injection, were matched to nonenhanced T1-weighted images obtained with similar sequence parameters.
CE T1-weighted Subtraction Maps
CE T1-weighted subtraction maps (Fig 1) were created by first performing linear registration between nonenhanced and CE T1-weighted images by using a 12–degree-of-freedom transformation and a correlation coefficient cost function in FSL (FLIRT; FMRIB Software Library, Oxford, England; http://www.fmrib.ox.ac.uk/fsl/). Although a 6–degree-of-freedom (ridged body) transformation was probably sufficient, a 12–degree-of-freedom transformation was chosen to provide a more robust framework to account for slight geometric distortions that may have occurred. Then, “Gaussian normalization” of image intensity for both nonenhanced and CE T1-weighted images was performed by using custom c-code and bash scripts, courtesy of the National Institutes of Mental Health Magnetoencephalography 3Core Facility (3dNormalize; NIMH MEG Core, Bethesda, Md; kurage.nimh.nih.gov/meglab/Med/3dNormalize), which normalizes image intensity by dividing each voxel by the standard deviation of the image intensity from the whole brain [SNor(x,y,z) = S (x,y,z)/σWB], where S is raw image signal intensity, Nor is normalized, x,y,z are voxel coordinates, and WB is whole brain. The use of a similar technique was recently shown to improve contrast-to-noise ratio and variability in relative cerebral blood volume maps, where signal intensity is relative (24). Next, voxel-by-voxel subtraction between normalized nonenhanced and CE T1-weighted images was performed. Image voxels with a positive (greater than zero) before-to-after change in normalized contrast enhancement signal intensity (ie, voxels increasing in MR signal after contrast agent administration) within T2-weighted fluid-attenuated inversion-recovery hyperintense regions were isolated to create the final CE T1-weighted subtraction maps and were summed to create estimates of tumor burden. CE T1-weighted subtraction maps were limited to T2-weighted fluid-attenuated inversion-recovery hyperintense regions to exclude large vessels and other hyperintense regions outside the primary tumor area.
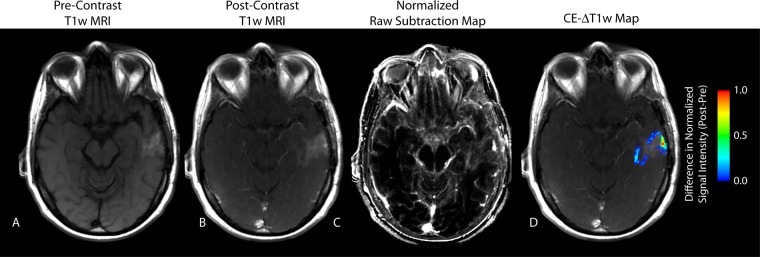
Figure 1:
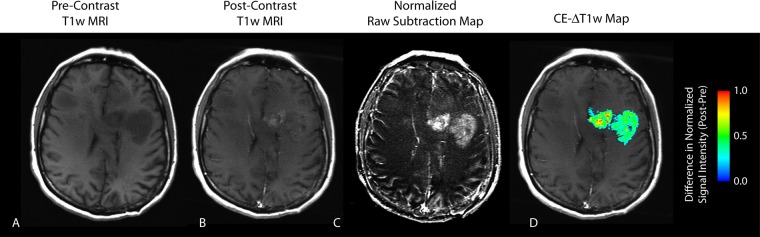
CE T1-weighted MR image (T1w MRI) in a 44-year-old female patient with recurrent GBM demonstrates an apparent reduction in contrast enhancement after bevacizumab therapy. A, Nonenhanced (Pre-Contrast) T1-weighted MR image. B, CE (Post-Contrast) T1-weighted MR image. C, Subtraction map. D, CE T1-weighted subtraction (CE-ΔT1w) map shows demarcation of an area of residual tumor identified as a positive increase in MR signal intensity after administration of contrast agent. Post-Pre = difference between after bevacizumab therapy and before bevacizumab therapy.
Conventional Segmentation
Conventional segmentation of contrast-enhancing regions on CE T1-weighted images involved isolation of hyperintense regions, excluding areas of obvious blood products and regions of central necrosis (hypointensity on CE T1-weighted images). The volume was calculated before and after the first treatment of bevacizumab by using a semiautomated procedure consisting of (a) manually defining the relative region of tumor occurrence, (b) determining thresholds on CE T1-weighted images within these regions by using an empirical threshold, and then (c) manually editing the resulting masks to exclude any nontumor tissue. Regions of interest were completed by using a combination of in-house section 510k (Food, Drug, and Cosmetic Act) Food and Drug Administration–cleared software and custom scripts in Analysis of Functional NeuroImages, or AFNI (National Institute of Mental Health, Bethesda, Md; http://afni.nimh.nih.gov/afni). Initial segmentation was performed by graduate research assistants (D.C.W., R.J.H., and J.N.C., with 2 years, 3 years, and 1 year of experience, respectively), and final segmented volumes were verified by an imaging scientist (B.M.E., with 5 years of experience) and a board-certified neuroradiologist (W.B.P., with 11 years of experience). All investigators involved in segmentation were blinded from survival data.
Definition of Tumor Progression
Tumor recurrence was determined at each institution by using the modified criteria of Macdonald et al (14), clear clinical progression in the absence of an MR imaging determination of progression, or death from any cause, whichever occurred first. (Further details are available at http://clinicaltrials.gov/show/NCT00345163.)
Statistical Analysis
Conventional segmentation and CE T1-weighted subtraction map estimates of enhancing volumes were compared at baseline and the first follow-up, along with the percentage change in volume between baseline and follow-up, by using the Student t test. PFS and OS for baseline biomarker assessment were calculated with respect to baseline image date, whereas landmark survival with respect to the first follow-up image date was used for assessment of posttreatment volumes and percentage change in volumes. Patients who had progression at time of first follow-up were excluded from prediction analysis. Log-rank analysis on Kaplan-Meier data and Cox proportional hazards regression models were used to understand the relationship between tumor size or change in size and survival. Patient data from a single institution (n = 80 patients, data not shown) were used as an exploratory training data set for defining specific thresholds for patient stratification in the BRAIN trial (ie, pretreatment volume of 15 cm3, posttreatment volume of 7.5 cm3, and decrease in volume of 25%) to set high- and low-risk groups for predicting PFS or OS. The least significant difference method of multiple comparisons was used to find this threshold in the exploratory training analysis. A log-rank test with the use of the Kaplan-Meier method and Cox proportional hazards regression models were used to predict PFS or OS for high- and low-risk groups. A log-rank test for trends was used to determine whether there was a trend between the volume of tumor and median PFS or OS by grouping patients according to tumor volume in increments of 5 cm3 for baseline and 2.5 cm3 for follow-up times. The two methods of analysis (conventional segmentation vs CE T1-weighted subtraction maps) and the interaction between them, along with the specific volume thresholds used for stratification, were tested by using a multivariate Cox proportional hazards regression model for PFS and OS. Receiver operating characteristic (ROC) analysis was performed by using the predefined volume thresholds to determine whether the segmentation techniques varied in their capability to help predict 6-month PFS, 6-month OS, and 12-month OS by using the area under the curve (AUC) as a measure of ROC performance. For all analyses, P < .05 was considered to indicate a significant difference. No adjustment for multiple comparisons was implemented when comparing pretreatment, posttreatment, and change in volume among the different measurement techniques. Statistical analyses were performed with software (Stata 12, 2011; College Station, Tex). All statistical analyses were performed by a single statistician (H.J.K., with more than 5 years of experience).
Results
Median PFS was 5.6 months (5.4 months from first follow-up), 25th quartile PFS was 2.8 months (1.9 months from first follow-up), and 75th quartile PFS was 11.1 months (11.4 months from first follow-up). Median OS was 9.2 months (8.6 months from first follow-up), 25th quartile OS was 5.6 months (5.0 months from first follow-up), and 75th quartile OS was 16.5 months (16.0 months from first follow-up). After bevacizumab treatment, in 126 of 160 BRAIN trial patients (79%), conventional segmentation revealed a measurable decrease in contrast-enhancing tumor volume, while in 131 of 160 BRAIN trial patients (82%), CE T1-weighted subtraction maps aided detection of a decrease in contrast-enhancing tumor. In general, the volume of contrast enhancement was larger when it was measured by using CE T1-weighted subtraction maps compared with conventional segmentation both before treatment (mean for conventional segmentation volume, 17.14 cm3 ± 1.20; mean for CE T1-weighted subtraction maps, 22.35 cm3 ± 1.47; P < .0001) and after treatment (mean for conventional segmentation volume, 8.13 cm3 ± 1.05; mean for CE T1-weighted subtraction maps, 10.52 cm3 ± 1.16; P < .0001). As illustrated in Figure 1, many of these patients had subtle, ill-defined regions of contrast enhancement after bevacizumab treatment, making it difficult to delineate the margins of the enhancing lesion. Qualitatively, the extent and boundaries of enhancing lesions were more easily demarcated on raw subtraction maps and subsequent CE T1-weighted subtraction maps compared with conventional CE T1-weighted images. On average, on raw subtraction images, contrast-to-noise ratio between enhancing tumor and surrounding normal-appearing tissue was increased by 56%, compared with that on standard CE T1-weighted images. As illustrated in Figure 2, patients in whom CE T1-weighted subtraction maps suggested lower tumor volumes than did conventional segmentation (approximately 20% of patients [n = 32]) demonstrated appreciable T1 shortening on nonenhanced T1-weighted images, resulting in overestimation of true enhancing lesion volume when we used only CE images for segmentation. Raw subtraction images and CE T1-weighted subtraction maps could then be used to isolate only the relevant enhancing tumor regions that had signal intensity increase after contrast agent administration beyond the effects of T1 shortening.
Figure 2:
Nonenhanced T1-weighted MR image in a 50-year-old male patient with recurrent GBM demonstrates significant T1 shortening after bevacizumab therapy, which may have been mistaken as residual tumoron CE T1-weighted MR image. A, Nonenhanced T1-weighted MR image. B, CE T1-weighted MR image. C, Subtraction map. D, CE T1-weighted subtraction map shows demarcation of an area of residual tumor identified as a positive increase in MR signal intensity after administration of contrast agent. Keys are the same as on Figure 1.
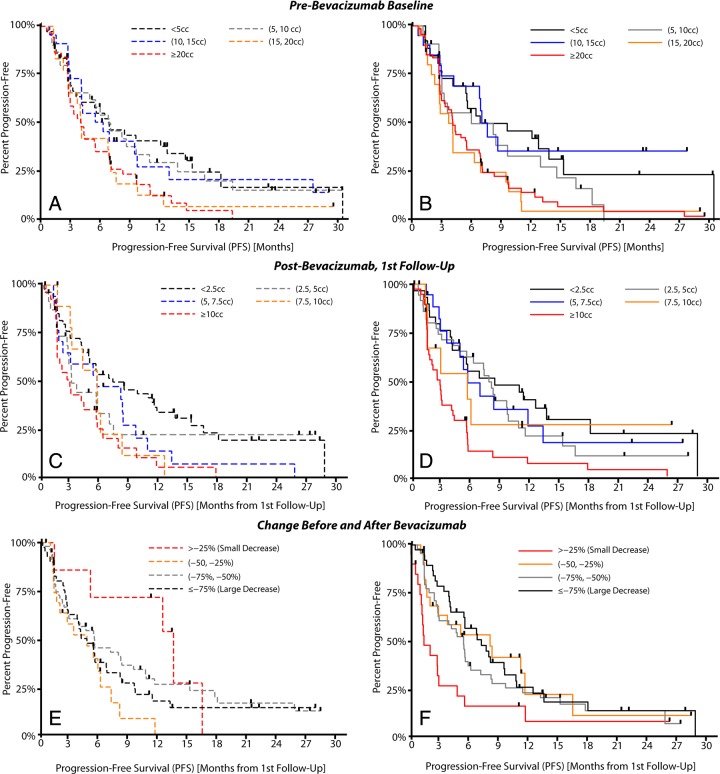
A strong linear trend was observed between pretreatment contrast-enhancing tumor volume and median PFS for both conventional segmentation (Fig 3) (P = .0042) and CE T1-weighted subtraction maps (P = .0022). Similar to pretreatment baseline trends, the volume of enhancing tumor at posttreatment follow-up times also demonstrated a significant trend with median PFS by using both conventional segmentation (P = .0002) and CE T1-weighted subtraction maps (P < .0001). Despite that both techniques demonstrated a similar trend, with decreasing tumor volumes having longer PFS, clearly increased separation between groups with respect to PFS both before and after bevacizumab treatment times was shown with CE T1-weighted maps. A clear trend was observed between the change in contrast-enhancing tumor volume and median PFS when we used CE T1-weighted subtraction maps (P = .0192), but not with conventional segmentation (P = .6234).
Figure 3:
Trends between tumor volume and PFS in the BRAIN trial for conventional segmentation (A, C, E) and CE T1-weighted subtraction maps (B, D, F). A, Trends in pretreatment tumor volume for conventional segmentation. B, Trends in pretreatment tumor volume for CE T1-weighted subtraction maps. C, Trends in posttreatment tumor volume for conventional segmentation. D, Trends in posttreatment tumor volume for CE T1-weighted subtraction maps. Tumor volume was measured in cubic centimeters (cc). E, Trends in volume change after therapy for conventional segmentation. F, Trends in volume change after therapy for CE T1-weighted subtraction maps.
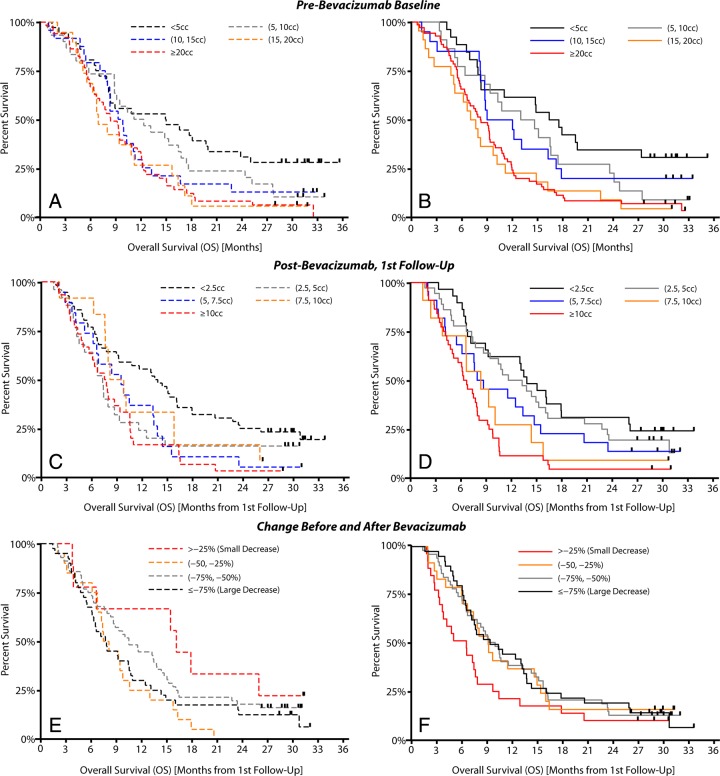
A strong linear trend was observed between the pretreatment volume of contrast enhancement and median OS for both conventional and CE T1-weighted subtraction map segmentation (Fig 4) (P = .0005 for conventional segmentation and P = .0001 for CE T1-weighted subtraction maps). The log-rank test for trends also indicated a trend between posttreatment tumor volume and median OS for both techniques (P = .0027 for conventional segmentation and P < .0001 for CE T1-weighted subtraction maps). Survival trends with respect to tumor volume and change in tumor volume after therapy were significant by using both CE T1-weighted subtraction maps and conventional segmentation; however, results for conventional segmentation were not intuitive. For example, although conventional segmentation technically showed a significant trend with respect to tumor volume groups, Figure 3, C, reveals that conventional segmentation did not demonstrate a linearly decreasing OS for increasing posttreatment tumor volume. Patients with the lowest tumor volume, less than 2.5 cm3, had the longest median OS, as expected; however, patients with a tumor volume of 5–7.5 cm3 or 7.5–10 cm3 had a moderate median OS, while, counterintuitively, patients with enhancing volumes between 2.5 cm3 and 5 cm3 had the shortest OS. On the other hand, tumor volumes estimated by using CE T1-weighted subtraction maps clearly demonstrated a linearly increasing median OS with increasing tumor volume (Fig 4, D). No trends were observed between the change in contrast-enhancing tumor volume after therapy and median OS for either technique (P = .3047 for conventional segmentation, P = .149 for CE T1-weighted subtraction maps). Interestingly, results by using conventional segmentation suggest that patients with a smaller decrease in enhancing tumor volume had the longer OS, whereas results obtained by using CE T1-weighted subtraction maps imply a more intuitive relationship between response and OS.
Figure 4:
Trends between tumor volume and OS in the BRAIN trial for conventional segmentation (A, C, E) and CE T1-weighted subtraction maps (B, D, F). A, Trends in pretreatment tumor volume for conventional segmentation. B, Trends in pretreatment tumor volume for CE T1-weighted subtraction maps. C, Trends in posttreatment tumor volume for conventional segmentation. D, Trends in posttreatment tumor volume for CE T1-weighted subtraction maps. Tumor volume was measured in cubic centimeters (cc). E, Trends in volume change after therapy for conventional segmentation. F, Trends in volume change after therapy for CE T1-weighted subtraction maps.
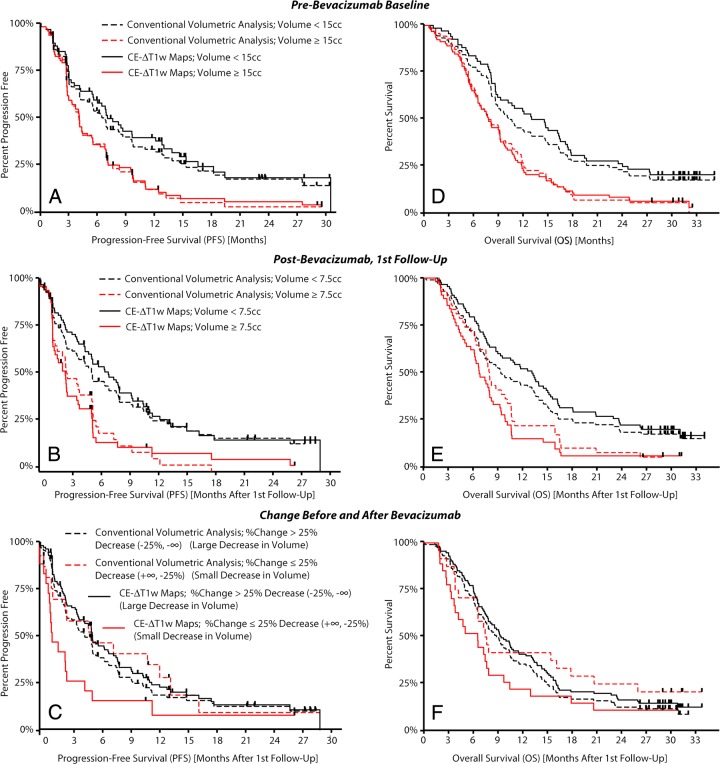
A pretreatment volume threshold of 15 cm3, a posttreatment volume threshold of 7.5 cm3, and a decrease in volume of greater than 25% were used to stratify high- and low-risk patients on the basis of training data from a single institution (80 patients, data not shown). Table E1 (online) summarizes the BRAIN trial survival analysis results with both techniques by using these thresholds. CE T1-weighted subtraction maps appeared to aid stratification of high- and low-risk patients better than did conventional segmentation in almost all scenarios (Fig 5). The multivariate Cox proportional hazards regression model suggested that CE T1-weighted subtraction maps had a significantly different hazard ratio compared with that of conventional segmentation when we examined the change in tumor volume after bevacizumab therapy in terms of PFS (P = .025), and trended to have a significantly different hazard ratio with respect to OS (P = .057). The change in tumor volume with conventional segmentation did not aid stratification of patient risk for PFS or OS, suggesting that patients having a larger decrease in enhancing tumor volume may be at a higher risk for progressing or expiring earlier than patients with smaller decreases in enhancement. Conventional segmentation did, however, significantly aid stratification of risk of PFS and OS in BRAIN trial patients when we examined pre- and posttreatment enhancing tumor volumes (Table E1 [online]): For PFS, the pretreatment P value was .003 and the posttreatment P value was .002; for OS, the pretreatment P value was .002 and the posttreatment P value was .041, but CE T1-weighted subtraction maps clearly better aided separation of high- and low-risk cohorts compared with conventional segmentation. The change in volume after bevacizumab treatment calculated by using CE T1-weighted subtraction maps significantly aided stratification of patients in terms of PFS (P = .004), but only trended to stratification of patients in terms of OS (P = .053).
Figure 5:
High- and low-risk patient stratification for the BRAIN trial with conventional segmentation or CE T1-weighted subtraction (CE-ΔT1w) maps. Patient cohorts were identified from single-stratification thresholds found in UCLA single-institution exploratory data (ie, pretreatment volume of 15 cm3 [cc], posttreatment volume of 7.5 cm3, and decrease in volume of 25%). A, PFS for high- and low-risk patients using pretreatment tumor volumes. B, PFS for high- and low-risk patients using posttreatment tumor volume estimates. C, PFS for high- and low-risk patients stratified on the basis of change in tumor volume as a result of therapy. D, OS for high- and low-risk patients by using pretreatment tumor volumes. E, OS for high- and low-risk patients by using posttreatment tumor volume estimates. F, OS for patients stratified on the basis of change in tumor volume after therapy.
ROC analysis applied to predefined clinical end points of 6-month PFS, 6-month OS, and 12-month OS demonstrated the added value of CE T1-weighted subtraction maps for lesion quantification. Although pretreatment tumor volumes quantified by using the two segmentation techniques resulted in similar ROC performance with respect to 6-month PFS (mean AUC for conventional vs CE T1-weighted subtraction maps, 0.58 ± 0.04 vs 0.60 ± 0.04; P = .3848), the volume of tumor burden on CE T1-weighted subtraction maps after therapy was clearly superior at aiding identification of patients who had progression at 6 months (mean AUC for conventional vs CE T1-weighted subtraction maps, 0.59 ± 0.04 vs 0.67 ± 0.04; P = .0294). Similarly, CE T1-weighted subtraction maps enhanced the ability to predict 6-month progression-free responders when we examined the percentage change in tumor volume (mean AUC for conventional vs CE T1-weighted subtraction maps, 0.49 ± 0.03 vs 0.58 ± 0.03; P = .0141). Similar to 6-month PFS, no difference in ROC performance was observed between conventional segmentation and CE T1-weighted subtraction maps with respect to 6-month OS for pretreatment tumor volume (mean AUC for conventional vs CE T1-weighted subtraction maps, 0.57 ± 0.04 vs 0.62 ± 0.04; P = .1624); however, CE T1-weighted subtraction maps clearly outperformed conventional segmentation when we used posttreatment tumor volumes (mean AUC for conventional vs CE T1-weighted subtraction maps, 0.52 ± 0.04 vs 0.61 ± 0.05; P = .0202) and the percentage change in tumor volume after therapy (mean AUC for conventional vs CE T1-weighted subtraction maps, 0.50 ± 0.04 vs 0.59 ± 0.04; P = .0226) to predict 6-month OS. When we used ROC analysis to examine the capability of CE T1-weighted subtraction maps and conventional segmentation to aid identification of 12-month survivors, the results indicated that CE T1-weighted subtraction maps were better when we used pretreatment tumor volumes (mean AUC for conventional vs CE T1-weighted subtraction maps, 0.59 ± 0.04 vs 0.66 ± 0.03; P = .0114), posttreatment tumor volumes (mean AUC for conventional vs CE T1-weighted subtraction maps, 0.60 ± 0.04 vs 0.69 ± 0.04; P = .0133) and trended toward better performance when we used the change in tumor volume (mean AUC for conventional vs CE T1-weighted subtraction maps, 0.48 ± 0.03 vs 0.56 ± 0.03; P = .0522). It is important to note that trends in the ROC plots were reversed between CE T1-weighted subtraction maps and conventional segmentation for change in tumor volume, where conventional segmentation counterintuitively suggested that patients who exhibited a large decrease in volume were more likely to have progression and expire sooner.
Discussion
To the best of our knowledge, results from the current study are the first to clearly identify a robust, repeatable relationship among tumor volumes, change in tumor volumes in response to bevacizumab therapy, and patient survival in recurrent GBM treated with bevacizumab in a multicenter clinical trial. Results from the current study demonstrate that detection and quantification of contrast-enhancing tumor burden in the context of bevacizumab therapy are greatly improved through the use of image subtraction of nonenhanced images from CE T1-weighted images (CE T1-weighted subtraction maps). Further, CE T1-weighted subtraction maps, or even raw subtraction maps, have the potential to aid oncologists and radiologists for traditional response assessment (ie, Response Assessment in Neuro-Oncology criteria [13] or criteria of Macdonald et al [14]).
CE T1-weighted subtraction maps appear to have a clear advantage over conventional segmentation in terms of lesion visualization and quantification after administration of bevacizumab. In most of the patients, subtle or ill-defined regions of residual contrast enhancement were present after bevacizumab treatment. Contrast-enhancing tumor regions are particularly challenging to demarcate from surrounding tissue when only traditional CE T1-weighted images are examined, inevitably leading to a high probability of inaccuracies when one quantifies enhancing tumor burden. In addition, approximately 20% of patients (n = 32) in the current study demonstrated appreciable T1 shortening after bevacizumab treatment, which generally led to overestimation of contrast-enhancing tumor volume by using conventional segmentation despite the attempt to avoid regions with known T1 shortening. This T1 shortening may be caused by spontaneous blood products or microbleeds within the lesion after antiangiogenic therapy, as bevacizumab and other antiangiogenic agents are known to increase the risk of venous thromboembolic events (25,26) and hemorrhage (5,23,27,28). CE T1-weighted subtraction maps were capable of helping to clearly define regions of contrast-enhancing tumor burden in both scenarios, whether the lesions were faint and diffusely enhancing on CE T1-weighted images or significant T1 shortening was present on nonenhanced T1-weighted images.
There were a few limitations to the current study that should be addressed. First, this study was retrospective and was based on the standard-of-care protocols at each site. Ideally, a standardized image acquisition protocol should be implemented to control various factors that may influence image contrast variation. MR receiver gain and coil sensitivity profiles should also be identical between nonenhanced and CE acquisitions to further limit signal intensity variation. In addition, contrast agent dose and the timing of CE T1-weighted images after contrast agent administration should be controlled. Also, the use of Gaussian normalization in the current study was chosen out of ease of implementation and recent evidence showing similar performance with other techniques (24), but other algorithms for image intensity normalization may perform better in this particular application. Last, while results from the current study demonstrate the capability of CE T1-weighted subraction maps to aid in the prediction of survival by using volumetric segmentation, these maps may also benefit in response evaluation by using conventional radiographic measures including the criteria of Macdonald et al (14) and the Response Assessment in Neuro-Oncology criteria (13). Future studies are still required to evaluate the possible added value of CE T1-weighted subtraction maps in terms of traditional response criteria.
In conclusion, multivariate survival analyses, visual observations, and ROC comparisons demonstrate that CE T1-weighted subtraction maps, compared with conventional segmentation of contrast-enhancing lesions, produce better stratification of high- and low-risk patients treated with bevacizumab and therefore should be used in future clinical trials involving evaluation of antiangiogenic therapies in brain tumors.
Advances in Knowledge
■ Volumetric analysis can aid prediction of survival in recurrent glioblastoma treated with bevacizumab (pretreatment volume, <15 cm3, P < .01; posttreatment volume, <7.5 cm3, P < .001).
■ The use of contrast-enhanced (CE) T1-weighted subtraction maps significantly improves the capability to quantify volume of enhancing tumor and aids prediction of survival after administration of bevacizumab.
Implication for Patient Care
■ The current study introduces a superior method for brain tumor delineation and quantification in the context of antiangiogenic therapy compared with conventional volumetric analysis applied to CE T1-weighted images directly, as demonstrated by using data from a multicenter clinical trial; use of this method will improve patient care by providing clinicians with a better tool for assessing patient response to antiangiogenic therapies compared with conventional volumetric analysis.
SUPPLEMENTAL TABLE
Received June 6, 2013; revision requested August 6; final revision received August 14; accepted September 6; final version accepted October 2.
B.M.E. supported by a UCLA Institute for Molecular Medicine Seed Grant, UCLA Radiology Exploratory Research Grant, University of California Cancer Research Coordinating Committee Grant, and ACRIN Young Investigator Initiative Grant. T.F.C. supported by Art of the Brain, the Ziering Family Foundation in memory of Sigi Ziering, Singleton Family Foundation, and Clarance Klein Fund for Neuro-Oncology.
Funding: This research was supported by the National Institutes of Health, National Cancer Institute (grant 1 R21 CA167354-01).
Clinical trial registration no. NCT00345163
Disclosures of Conflicts of Interest: B.M.E. No relevant conflicts of interest to disclose. H.J.K. No relevant conflicts of interest to disclose. D.C.W. No relevant conflicts of interest to disclose. W.B.P. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: received personal fees from Genentech and Roche. Other relationships: none to disclose. J.N.C. No relevant conflicts of interest to disclose. R.J.H. No relevant conflicts of interest to disclose. A.L. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: received a grant and personal fees from Genentech. Other relationships: none to disclose. P.L.N. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: served on the advisory board of Genentech. Other relationships: none to disclose. T.F.C. Financial activities related to the present article: received personal fees from Roche and Genentech. Financial activities not related to the present article: none to disclose. Other relationships: none to disclose.
Abbreviations:
- AUC
- area under the curve
- CE
- contrast enhanced
- GBM
- glioblastoma
- OS
- overall survival
- PFS
- progression-free survival
- ROC
- receiver operating characteristic
References
- 1.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 2007;25(30):4722–4729. [DOI] [PubMed] [Google Scholar]
- 2.Chamberlain MC. Bevacizumab plus irinotecan in recurrent glioblastoma [letter]. J Clin Oncol 2008;26(6):1012–1013. [DOI] [PubMed] [Google Scholar]
- 3.Vrendenburg JJ, Desjardins A, Reardon DA, Friedman HS. Reply. J Clin Oncol 2008;26(6):1013. [Google Scholar]
- 4.Schiff D, Purow B. Bevacizumab in combination with irinotecan for patients with recurrent glioblastoma multiforme. Nat Clin Pract Oncol 2008;5(4):186–187. [DOI] [PubMed] [Google Scholar]
- 5.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology 2008;70(10):779–787. [DOI] [PubMed] [Google Scholar]
- 6.Ananthnarayan S, Bahng J, Roring J, et al. Time course of imaging changes of GBM during extended bevacizumab treatment. J Neurooncol 2008;88(3):339–347. [DOI] [PubMed] [Google Scholar]
- 7.Norden AD, Drappatz J, Wen PY. Antiangiogenic therapies for high-grade glioma. Nat Rev Neurol 2009;5(11):610–620. [DOI] [PubMed] [Google Scholar]
- 8.Lucio-Eterovic AK, Piao Y, de Groot JF. Mediators of glioblastoma resistance and invasion during antivascular endothelial growth factor therapy. Clin Cancer Res 2009;15(14):4589–4599. [DOI] [PubMed] [Google Scholar]
- 9.de Groot JF, Fuller G, Kumar AJ, et al. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro-oncol 2010;12(3):233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chamberlain MC. Radiographic patterns of relapse in glioblastoma. J Neurooncol 2011;101(2):319–323. [DOI] [PubMed] [Google Scholar]
- 11.Keunen O, Johansson M, Oudin A, et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci U S A 2011;108(9):3749–3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norden AD, Drappatz J, Wen PY. Novel anti-angiogenic therapies for malignant gliomas. Lancet Neurol 2008;7(12):1152–1160. [DOI] [PubMed] [Google Scholar]
- 13.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 14.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 15.Ellingson BM, Cloughesy TF, Lai A, Nghiemphu PL, Mischel PS, Pope WB. Quantitative volumetric analysis of conventional MRI response in recurrent glioblastoma treated with bevacizumab. Neuro-oncol 2011;13(4):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bedekar DP, Jensen T, Rand SD, Malkin MG, Connelly J, Schmainda KM. Delta T1 method: an automatic post-contrast ROI selection technique for brain tumors [abstr]. In: Proceedings of the Eighteenth Meeting of the International Society for Magnetic Resonance in Medicine. Berkeley, Calif: International Society for Magnetic Resonance in Medicine, 2010; 2166. [Google Scholar]
- 17.Bedekar DP, Schmainda KM, Rand SD, et al. Delta T1 (dT1) method as a tool to evaluate tumor progression in patients with brain cancer. J Clin Oncol 2011;29(suppl): e21056. [Google Scholar]
- 18.Harrington DP, Boxt LM, Murray PD. Digital subtraction angiography: overview of technical principles. AJR Am J Roentgenol 1982;139(4):781–786. [DOI] [PubMed] [Google Scholar]
- 19.Gauvrit JY, Leclerc X, Oppenheim C, et al. Three-dimensional dynamic MR digital subtraction angiography using sensitivity encoding for the evaluation of intracranial arteriovenous malformations: a preliminary study. AJNR Am J Neuroradiol 2005;26(6):1525–1531. [PMC free article] [PubMed] [Google Scholar]
- 20.Tatli S, Acar M, Tuncali K, Sadow CA, Morrison PR, Silverman SG. MRI assessment of percutaneous ablation of liver tumors: value of subtraction images. J Magn Reson Imaging 2013;37(2):407–413. [DOI] [PubMed] [Google Scholar]
- 21.An C, Park MS, Kim D, et al. Added value of subtraction imaging in detecting arterial enhancement in small (<3 cm) hepatic nodules on dynamic contrast-enhanced MRI in patients at high risk of hepatocellular carcinoma. Eur Radiol 2013;23(4):924–930. [DOI] [PubMed] [Google Scholar]
- 22.Ogura A, Hayakawa K, Yoshida S, Maeda F, Saeki F, Syukutani A. Use of dynamic phase subtraction (DPS) map in dynamic contrast-enhanced MRI of the breast. J Comput Assist Tomogr 2011;35(6):749–752. [DOI] [PubMed] [Google Scholar]
- 23.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 24.Ellingson BM, Zaw T, Cloughesy TF, et al. Comparison between intensity normalization techniques for dynamic susceptibility contrast (DSC)-MRI estimates of cerebral blood volume (CBV) in human gliomas. J Magn Reson Imaging 2012;35(6):1472–1477. [DOI] [PubMed] [Google Scholar]
- 25.Zangari M, Fink LM, Elice F, Zhan F, Adcock DM, Tricot GJ. Thrombotic events in patients with cancer receiving antiangiogenesis agents. J Clin Oncol 2009;27(29):4865–4873. [DOI] [PubMed] [Google Scholar]
- 26.Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA 2008;300(19):2277–2285. [DOI] [PubMed] [Google Scholar]
- 27.Lai A, Tran A, Nghiemphu PL, et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol 2011;29(2):142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raizer JJ, Grimm S, Chamberlain MC, et al. A phase 2 trial of single-agent bevacizumab given in an every-3-week schedule for patients with recurrent high-grade gliomas. Cancer 2010;116(22):5297–5305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.