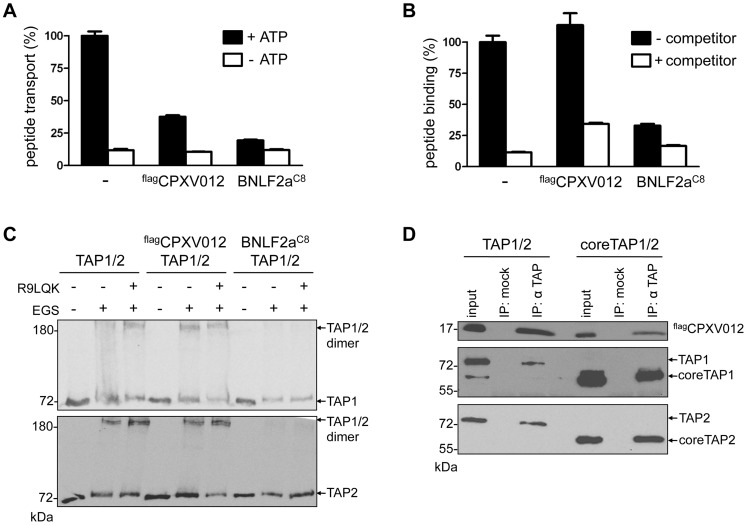
Figure 2. CPXV012 inhibits peptide transport but not peptide binding to TAP.
TAP1, TAP2, flagCPXV012, and BNLF2aC8 were coexpressed in Sf9 cells as indicated. (A) CPXV012 inhibits peptide transport of TAP. Crude membranes were incubated with RRYQNSTC(F)L peptide (C(F), fluorescein-labeled cysteine; N-core glycosylation site underlined) in the absence or presence of ATP. Translocated and N-core glycosylated peptides were bound to ConA-beads and quantified by fluorescence. Peptide transport by TAP was normalized to 100%. The means of at least three independent experiments are shown. Error bars indicate the S.D. (B) CPXV012 does not inhibit peptide binding to TAP. Crude membranes were incubated with high-affinity peptide RRYC(F)KSTEL (filled bars). A 100-fold excess of RRYQKSTEL (R9LQK) was used to probe for unspecific binding (open bars). Bound peptides were quantified by fluorescence. The membranes used for (A) and (B) expressed similar amounts of TAP1/2 (S1 Figure). (C) CPXV012 does not interfere with a peptide-induced conformational change of TAP. Membranes were incubated in the presence or absence of the peptide R9LQK. After EGS cross-linking, samples were analyzed by SDS-PAGE (6%) and immunoblotting with TAP1 (mAb 148.3) and TAP2 (mAb 435.3) specific antibodies. (D) CPXV012 binds to coreTAP. flagCPXV012 was coexpressed with either full-length TAP1/2 (left) or coreTAP1/2 (right). Proteins were immunoprecipitated with a mixture of polyclonal TAP1 (1p2) and TAP2 specific (2p4) antibodies (IP). The anti-US6 antibody was used a negative control (mock). Samples were analyzed by SDS-PAGE (12%) and subsequent immunoblotting with either anti-flag, monoclonal anti-TAP1 (mAb 148.3), or anti-TAP2 (mAb 435.3) antibodies. An aliquot (1/20) of the crude membrane input is shown.