Abstract
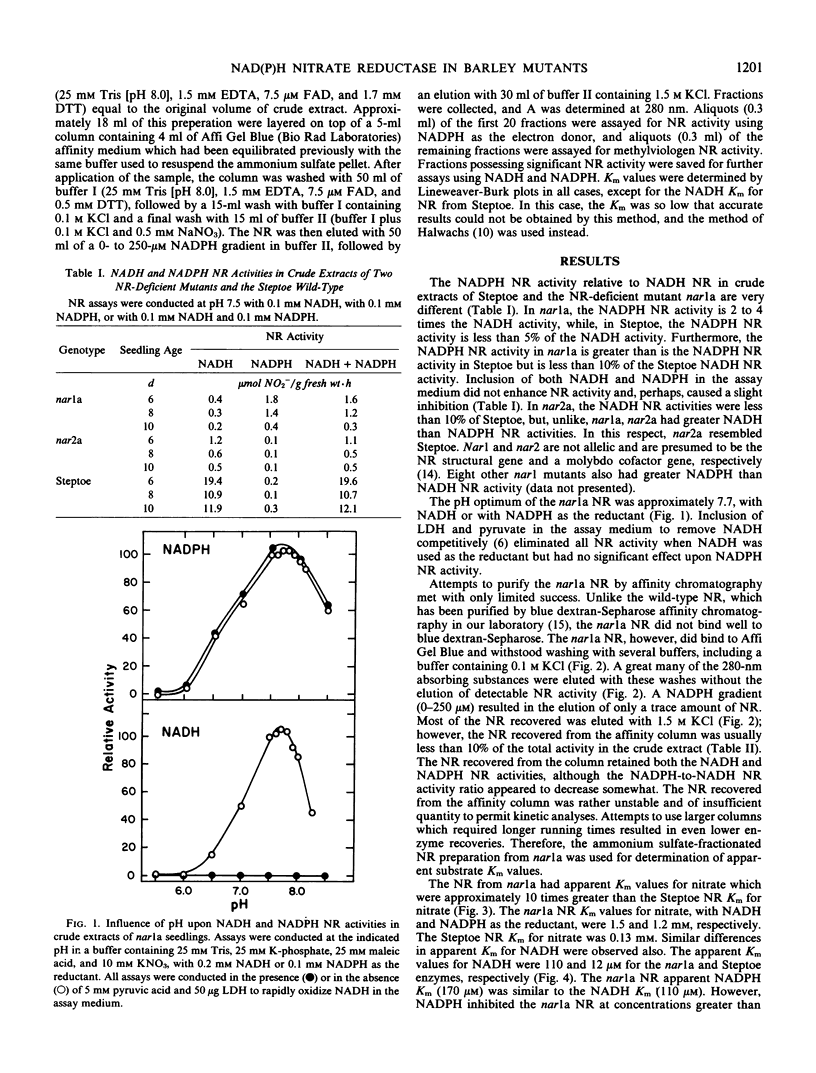
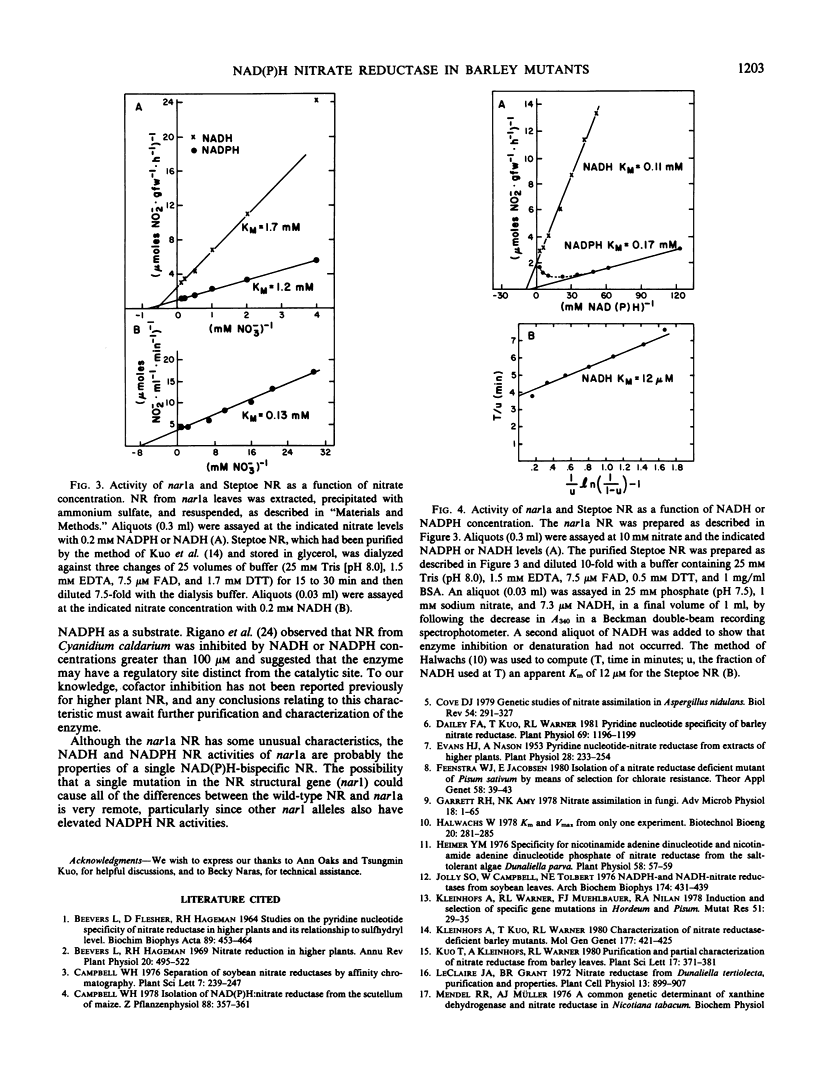
A barley (Hordeum vulgare L.) mutant, nar1a (formerly Az12), deficient in NADH nitrate reductase activity is, nevertheless, capable of growth with nitrate as the sole nitrogen source. In an attempt to identify the mechanism(s) of nitrate reduction in the mutant, nitrate reductase from nar1a was characterized to determine whether the residual activity is due to a leaky mutation or to the presence of a second nitrate reductase. The results obtained indicate that the nitrate reductase in nar1a differs from the wild-type enzyme in several important aspects. The pH optima for both the NADH and the NADPH nitrate reductase activities from nar1a were approximately pH 7.7, which is slightly greater than the pH 7.5 optimum for the NADH activity and considerably greater than the pH 6.0 to 6.5 optimum for the NADPH activity of the wild-type enzyme. The nitrate reductase from nar1a exhibits greater NADPH than NADH activity and has apparent Km values for nitrate and NADH that are approximately 10 times greater than those of the wild-type enzyme. The nar1a nitrate reductase has apparent Km values of 170 micromolar for NADPH and 110 micromolar for NADH. NADPH, but not NADH, inhibited the enzyme at concentrations greater than 50 micromolar.
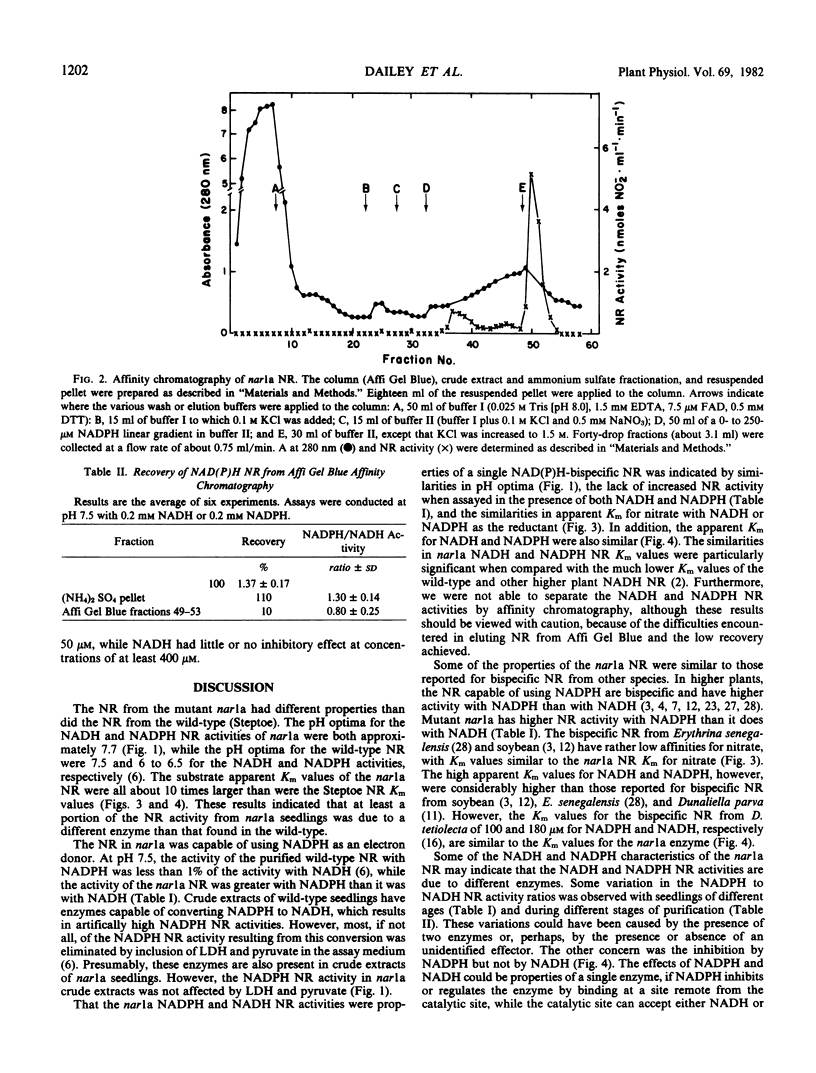
Unlike that of the wild-type, the nitrate reductase from nar1a did not bind to blue dextran-Sepharose. The nar1a enzyme did bind to Affi Gel Blue, but recoveries were low. The NADH and NADPH nitrate reductase activities of nar1a were not separated by affinity chromatography. The nitrate reductase in nar1a is a different enzyme than the wild-type NADH nitrate reductase and appears to be a NAD(P)H-bispecific enzyme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEEVERS L., FLESHER D., HAGEMAN R. H. STUDIES ON THE PYRIDINE NUCLEOTIDE SPECIFICITY OF NITRATE REDUCTASE IN HIGHER PLANTS AND ITS RELATIONSHIP TO SULFHYDRYL LEVEL. Biochim Biophys Acta. 1964 Sep 18;89:453–464. doi: 10.1016/0926-6569(64)90071-9. [DOI] [PubMed] [Google Scholar]
- Cove D. J. Genetic studies of nitrate assimilation in Aspergillus nidulans. Biol Rev Camb Philos Soc. 1979 Aug;54(3):291–327. doi: 10.1111/j.1469-185x.1979.tb01014.x. [DOI] [PubMed] [Google Scholar]
- Dailey F. A., Kuo T., Warner R. L. Pyridine nucleotide specificity of barley nitrate reductase. Plant Physiol. 1982 May;69(5):1196–1199. doi: 10.1104/pp.69.5.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans H. J., Nason A. Pyridine Nucleotide-Nitrate Reductase from Extracts of Higher Plants. Plant Physiol. 1953 Apr;28(2):233–254. doi: 10.1104/pp.28.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett R. H., Amy N. K. Nitrate assimilation in fungi. Adv Microb Physiol. 1978;18:1–65. doi: 10.1016/s0065-2911(08)60414-2. [DOI] [PubMed] [Google Scholar]
- Heimer Y. M. Specificity for Nicotinamide Adenine Dinucleotide and Nicotinamide Adenine Dinucleotide Phosphate of Nitrate Reductase from the Salt-tolerant Alga Dunaliella parva. Plant Physiol. 1976 Jul;58(1):57–59. doi: 10.1104/pp.58.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly S. O., Campbell W., Tolbert N. E. NADPH- and NADH-nitrate reductases from soybean leaves. Arch Biochem Biophys. 1976 Jun;174(2):431–439. doi: 10.1016/0003-9861(76)90371-4. [DOI] [PubMed] [Google Scholar]
- Orihuel-Iranzo B., Campbell W. H. Development of NAD(P)H: and NADH:Nitrate Reductase Activities in Soybean Cotyledons. Plant Physiol. 1980 Apr;65(4):595–599. doi: 10.1104/pp.65.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigano C., Violante U., Aliotta G. Kinetic aspects of nitrate reductase from Cyanidium caldarium. Inhibition by reduced pyridine nucleotides. Biochim Biophys Acta. 1973 Nov 15;327(1):19–23. doi: 10.1016/0005-2744(73)90098-3. [DOI] [PubMed] [Google Scholar]
- Scholl R. L., Harper J. E., Hageman R. H. Improvements of the nitrite color development in assays of nitrate reductase by phenazine methosulfate and zinc acetate. Plant Physiol. 1974 Jun;53(6):825–828. doi: 10.1104/pp.53.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn D. R., Carr P. W., Klatt L. N. Minimization of a sodium dithionite-derived interference in nitrate reductase-methyl viologen reactions. Anal Biochem. 1976 Oct;75(2):464–471. doi: 10.1016/0003-2697(76)90101-9. [DOI] [PubMed] [Google Scholar]
- Shen T. C., Funkhouser E. A., Guerrero M. G. NADH- and NAD(P)H-Nitrate Reductases in Rice Seedlings. Plant Physiol. 1976 Sep;58(3):292–294. doi: 10.1104/pp.58.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner R. L. Nitrate Utilization by Nitrate Reductase-deficient Barley Mutants. Plant Physiol. 1981 Apr;67(4):740–743. doi: 10.1104/pp.67.4.740. [DOI] [PMC free article] [PubMed] [Google Scholar]


