FIGURE 6.
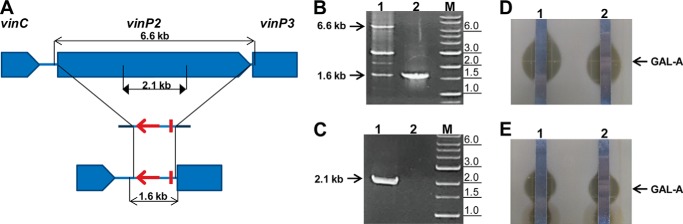
Targeted deletion scheme (A), PCR confirmation (B and C), and TLC-coupled antifungal assay (D and E) of the vinP2 mutant. A, the relevant genetic organization of the WT gene (top) and the vinP2 deletion mutant (bottom) are shown with a simple representation of the cognate gene inactivation construct (middle). For simplicity, only the target gene is shown for each gene inactivation construct. aacIV and oriT in the PCR targeting cassette are denote by a bold arrow and bar, respectively. PCR primer positions are shown along with the estimated sizes of the PCR products. B and C, agarose gel electrophoretic analysis of the PCR products from total DNA samples. The primer pairs are complementary to the region outside (B) or inside (C) the deletion: lane 1, WT; lane 2, the vinP2::aacIV mutant; lane M, DNA size marker. B, the 1.6-kb band in WT resulted from nonspecific PCR amplification; the PCR experiment was performed in a low stringency condition for the amplification of the 6.6-kb fragment in WT. A, the PCR strategy is shown, with triangular arrows indicating the target fragment inside the deletion. Thus, no PCR amplifications should be observed for the deletion mutant (C). The primer pairs used in this analytical PCR are vingpo-F/R (B) and vingne-F/R (C) (Table 1). The DNA size marker contains DNA fragments of 0.1, 0.2, 0.3, 0.4, 0.5, 0.65, 0.8, 1.0, 1.5, 2.0, 3.0, 4.0, 5.0, 6.0, 8.0, and 10.0 kb. The estimated sizes of the PCR products are indicated on the left by arrows, and the relevant DNA size marker fragments are indicated on the right in kb. D and E, the TLC-coupled antifungal assay against C. neoformans was performed with ethyl acetate extract of the culture supernatant (D) and methanol extract of the mycelium (E). The position of GAL-A is indicated by arrows.
