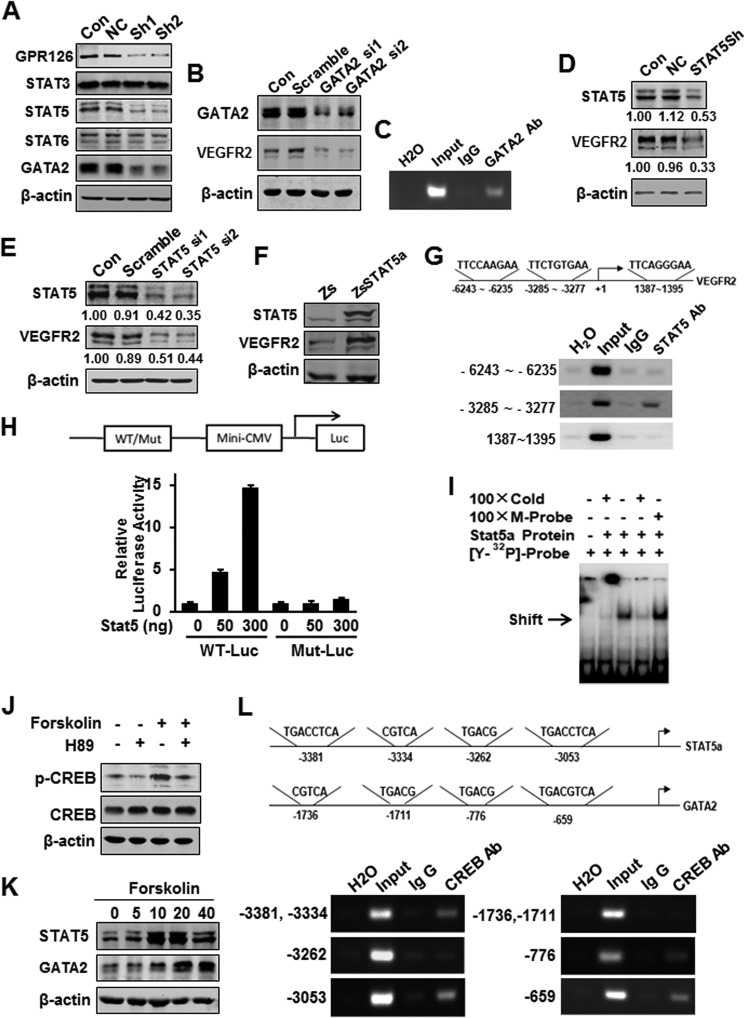
FIGURE 6.
GPR126 stimulates VEGFR2 transcription via STAT5 and GATA2. A, effects of GPR126 on the expression of transcription factors involved in angiogenesis. Protein levels were analyzed by immunoblot using specific antibodies in HMEC-1 cells (Con) and in cells infected with vector control (NC) or GPR126 shRNA (Sh1 and Sh2). B and C, regulation of VEGFR2 expression by GATA2. VEGFR2 protein level was analyzed by immunoblot in HMEC-1 cells (Con), cells transfected with control siRNA (Scramble), or GATA2 siRNA (GATA2 si1 and GATA2 si2) (B). Chromatin of HMEC-1 cells was immunoprecipitated with anti-GATA2 antibody or IgG control. The extracted DNA was used for PCR amplifications with VEGFR2 promoter-specific primers (C). D–F, regulation of VEGFR2 expression by STAT5. VEGFR2 protein level was analyzed by immunoblot in HMEC-1 cells (Con), cells transfected with control shRNA (NC), or STAT5 shRNA vector (STAT5Sh) (D), or control siRNA (Scramble), and STAT5 siRNA (ST5 si1 and ST5 si2) (E), respectively. F, forced expression of STAT5 increased VEGFR2 expression in HMEC-1 cells. Zs, control vector; ZsSTAT5, STAT5 overexpression vector. G, chromatin of HMEC-1 cells was immunoprecipitated with anti-STAT5 antibody or IgG control. The extracted DNA was used for PCR amplifications with VEGFR2 promoter-specific primers. STAT5 strongly bound to the predicted site (−3285 to −3277, TTCTGTGAA) in the VEGFR2 promoter. The assays were conducted at least three times. H, wild type (WT-Luc) or STAT5-binding site mutant (Mut-Luc) VEGFR2 promoter luciferase reporter was transfected with increasing doses of STAT5 expression plasmid, and the luciferase activity was determined. I, in vitro translated STAT5 protein was incubated with hot STAT5-binding element derived from VEGFR2 for EMSAs. Cold WT (Cold) or mutant STAT5-binding site (M-probe) probes were subjected for competition. J–L, GPR126 regulated STAT5 and GATA2 expression through cAMP-activated PKA-CREB pathway. Phospho-CREB (Ser-133) was activated in HMEC-1 cell with forskolin (20 μm) stimulation and inhibited with H-89 (10 μm) treatment (J). K, forskolin increased the STAT5 and GATA2 protein levels in a dose-dependent manner in HMEC-1 cells. L, ChIP assays of the CRE site in the STAT5 and GATA2 promoter. Top panel showed the predicted conserved CRE site or half-CRE site in the STAT5 promoter. Middle panel showed that of the GATA2 promoter. After immunoprecipitation of the cross-linked complexes, DNA was recovered by phenol/chloroform extraction. Then the DNA were amplified by PCR using indicated primers. The PCR bands in bottom panel showed the DNA fragment precipitated by anti-CREB antibody in the promoter region of STAT5 (bottom left) and GATA2 (bottom right), respectively.