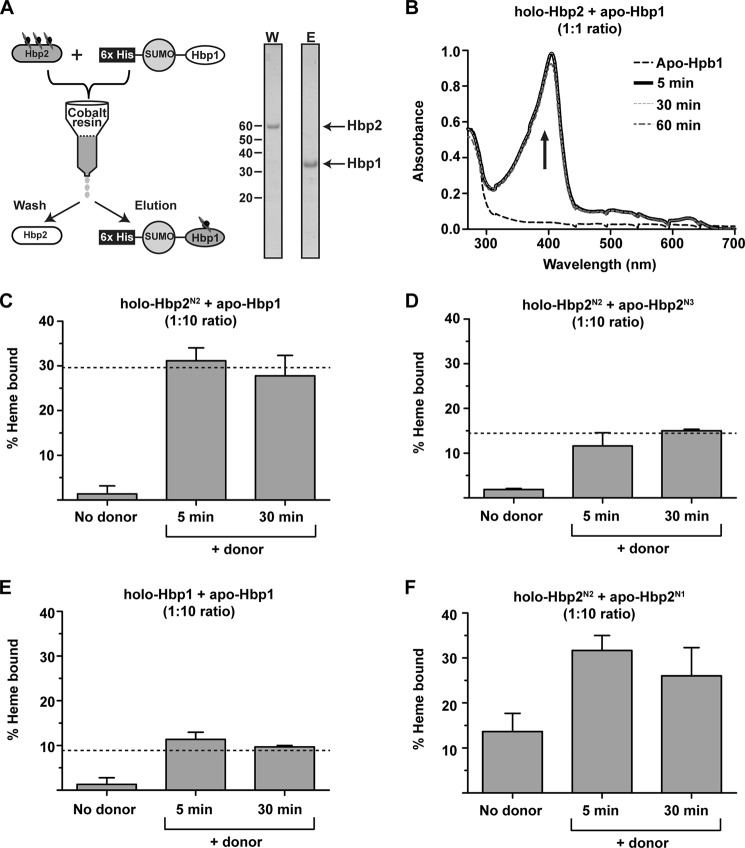
FIGURE 3.
Demonstration of hemin transfer. A, a schematic of the hemin transfer experiment. A hemin donor (e.g. holo-Hbp2) is mixed with a histidine-tagged hemin acceptor (e.g. His6-SUMO-Hbp1). The proteins are then separated using a Co2+-chelating column, and the amount of hemin transferred to the acceptor is determined. A representative SDS-polyacrylamide gel shows complete separation of Hbp2 and His6-SUMO-Hbp1 after incubation for 60 min. W, wash; E, elution. B, hemin transfer from Hbp2 and Hbp1. The UV spectrum of the imidazole eluted His6-SUMO-Hbp1 protein before and after incubation with hemin-loaded Holo-Hbp2 (1:1 stoichiometry). Transfer from Hbp2 to His6-SUMO-Hbp1 is complete within 5 min of mixing the proteins. C, hemin transfer from holo-Hbp2N2 to His6-SUMO-Hbp1 (1:10 stoichiometry). The experiments were performed as shown in A. The amount of hemin captured by His6-SUMO-Hbp1 was determined by measuring the ASoret/A280 ratio of the eluted fractions, which was then divided by the ratio representing heme-saturated protein to determine the fraction of protein bound with hemin. The dashed line indicates the expected level of hemin saturation of the acceptor based on the dissociation constants measured by ITC. D, as in C but hemin transfer from holo-Hbp2N2 to His6-SUMO-Hbp2N3 was measured. E, as in C but hemin transfer from holo-Hbp1 to His6-SUMO-Hbp1 was measured. F, as in C but hemin transfer from holo-Hbp2N2 to His6-SUMO-Hbp2N1 was measured. There is no dashed line as ITC was not used to determine the hemin affinity of Hbp2N1. Error bars represent S.D. from three experiments.