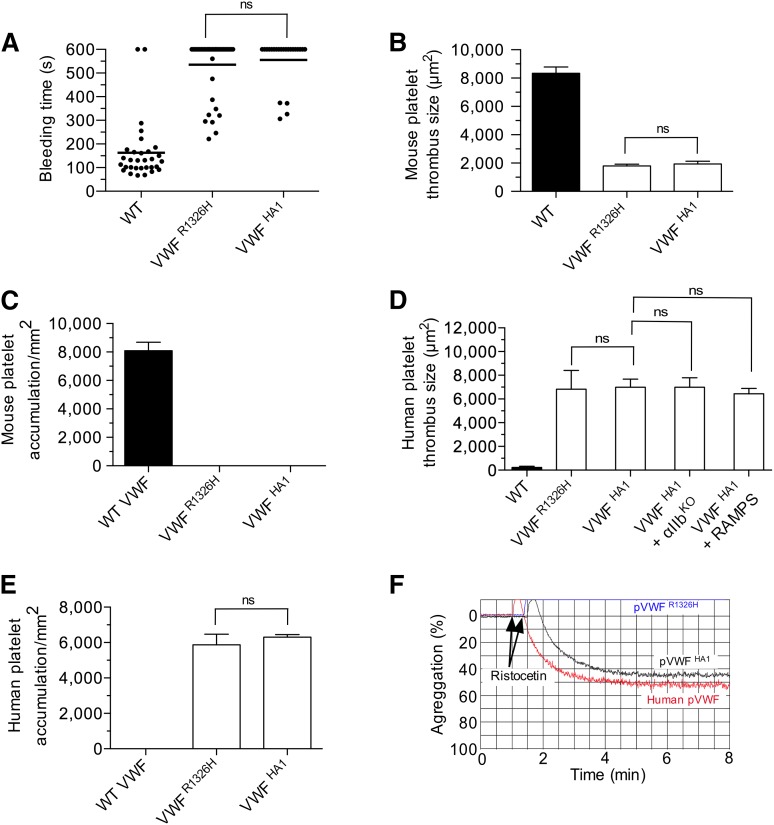
Figure 5.
Hemostatic and thrombotic properties of plasma VWFHA1. (A) Tail bleeding times for WT, VWFR1326H, and VWFHA1 mice. Each point represents 1 individual animal; lines show the mean of each group. (B) Thrombus formation in laser-injured arterioles of WT and VWF mutant mice (n = 7 mice per genotype; 1 arteriole per animal). Fluorescent images were obtained using a 20× water-immersion objective, a Yokogawa CSU-22 spinning disk confocal scanner, and a 561 nm laser line to detect rhodamine-labeled mouse platelets. The extent of thrombus formation was assessed for 2 minutes postinjury and its area determined by off-line analysis (Image IQ and Image-Pro Plus). (C) Accumulation of mouse platelets on surface-immobilized WT or mutant plasma VWF at a WSR of 1,600 s−1 (n = 3). Images were obtained using a CCD camera and analyzed using Image-Pro Plus. (D) Human platelet-mediated thrombus formation in laser injured arterioles of WT or VWF mutant animals (n = 7 mice per genotype; 1 arteriole per animal). A 488 nm laser line was used to detect BCECF-labeled human platelets. Rabbit anti-mouse platelet serum (RAMPS) was used to deplete mouse platelets. (E) Accumulation of human platelets on surface-immobilized WT or mutant plasma VWF at a WSR of 1,600 s−1 (n = 3). (F) Representative plot depicting the extent of ristocetin-induced aggregation of lyophilized human platelets in plasma from human volunteers or VWF mutant animals (n = 2 experiments performed in duplicate). Data are the mean ± SD. ns, not significant (P > .05).