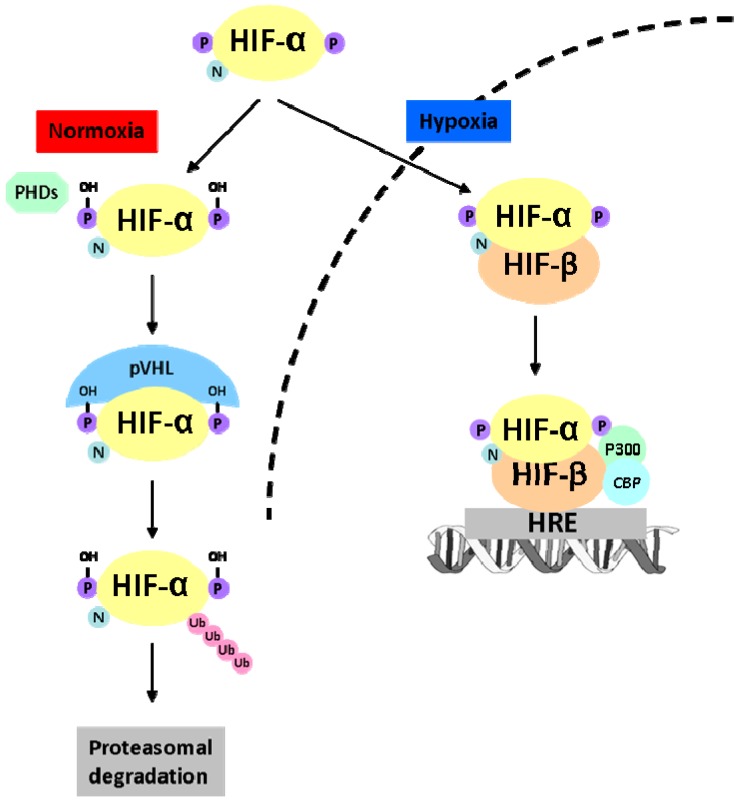
Figure 3.
A schematic representation of the oxygen-dependent regulation of HIF-α. During normoxia, HIF-α is hydroxylated by prolyl hydroxylase domain proteins (PHDs) on proline residues. These proline residues are recognized by the protein Von Hippel Lindau (pVHL), which results in proteasomal degradation. Upon hypoxia, HIF-α is not hydroxylated and subsequently translocated to the nucleus. In the nucleus, HIF-α heterodimerizes with HIF-β; this allows the co-factors p300 and Creb-binding protein (CBP) to bind to the heterodimer. The complex binds to the hypoxia-responsive elements (HRE) and thereby induces gene expression. Adapted from [60].