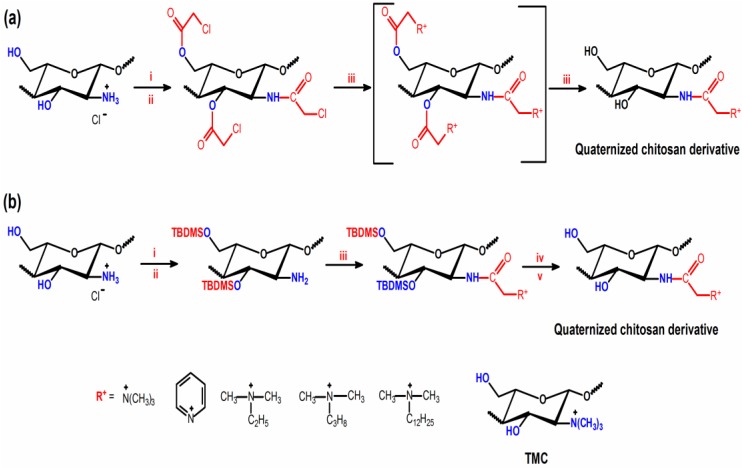
Scheme 12.
Route for synthesis of the N-quaternized chitosan derivatives. Reagents and conditions: (a) (i) TEA, pyridine, H2O; (ii) chloracetyl chloride, TEA, DMF, N2 atmosphere, 72 h, 22 °C; (iii) tertiary amine, DMF or NMP or pyridine, N2 atmosphere, NaI as catalyst when TEA and tripropylamine as reagents, 72 h, 60 °C, ion exchanged, dialysis; and (b) (i) methansulfonic acid, H2O; (ii) TBDMSCl, imidazole, DMSO, N2 atmosphere, 0 °C, 20 min, 2 × 24 h, 22 °C; (iii) chloracetyl chloride, TEA, pyridine dichloromethane, 1 h, 0 °C; (iv) dimethyldodecylamine or dimethylbutylamine, chloroform, 40 h, 22 °C; and (v) concentration of HCl, ethanol, 6 h, 22 °C [76,88].