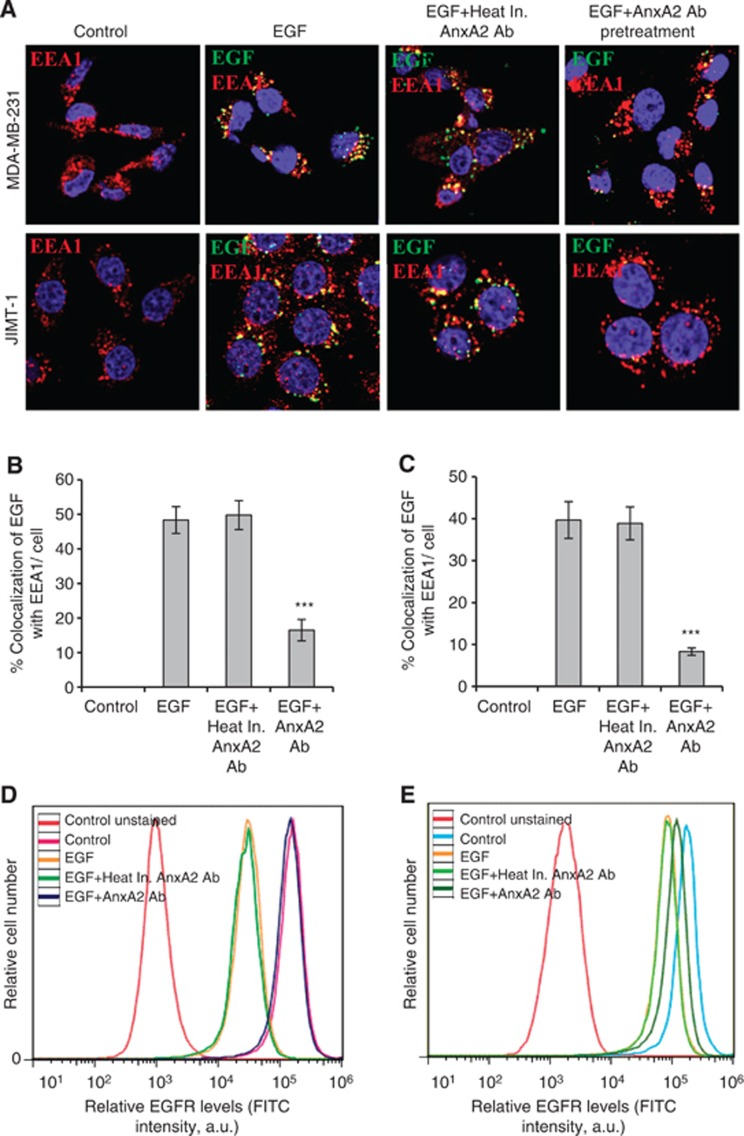
Figure 5.
AnxA2 antibody inhibits the EGF-induced EGFR internalisation in MDA-MB-231 and JIMT-1 cells. (A) The membrane function of AnxA2 was blocked by the treatment of AnxA2 (D1/274.5) antibody (2 μg ml−1) for 2 h after 12 h of serum starvation. MDA-MB-231 and JIMT-1 cells were treated with or without FITC labelled EGF (50 ng ml−1) for 20 min after AnxA2 antibody pretreatment. The cells were fixed, permeabilised, and incubated with rabbit anti-EEA1 antibody followed by anti-rabbit Alexa 595 secondary antibody. The slides were examined using LSM 510 Meta confocal system. The merged pictures of EGF (green), EEA1 (red) and nucleus (blue) are representative images of three independent experiments. Yellow dots represent the colocalisation of green and red colors, which indicates that EGF is colocalised with EEA1. Quantitative analysis of the percentage of colocalisation of EGF with EEA1 in MDA-MB-231 (B) and JIMT-1 (C) was calculated by analyzing a minimum of 10 cells from each treatment randomly taken from two independent experiments (***P<0.001 vs EGF or EGF+Heat inactivated AnxA2 antibody pretreatment). Inhibition of EGF-induced internalisation of EGFR at cell surface by AnxA2 antibody was measured by flow cytometry in MDA-MB-231 (D) and JIMT-1 (E) cells. The cells were incubated with or without EGF (50 ng ml−1) for 5 min after 2 h of heat inactivated AnxA2 (D1/274.5) antibody (2 μg ml−1) or AnxA2 (D1/274.5) antibody (2 μg ml−1) pretreatment. After fixation, cells were stained with anti-EGFR antibody without permeabilisation, followed by Alexa fluor 488-conjugated anti-rabbit IgG. After washing, cells were analysed by flow cytometry using the FITC signal detector (FL1) in Beckman Coulter FC500 flow cytometer. The data are plotted for cell numbers vs the intensity of fluorescence. Results are representative of two independent experiments.