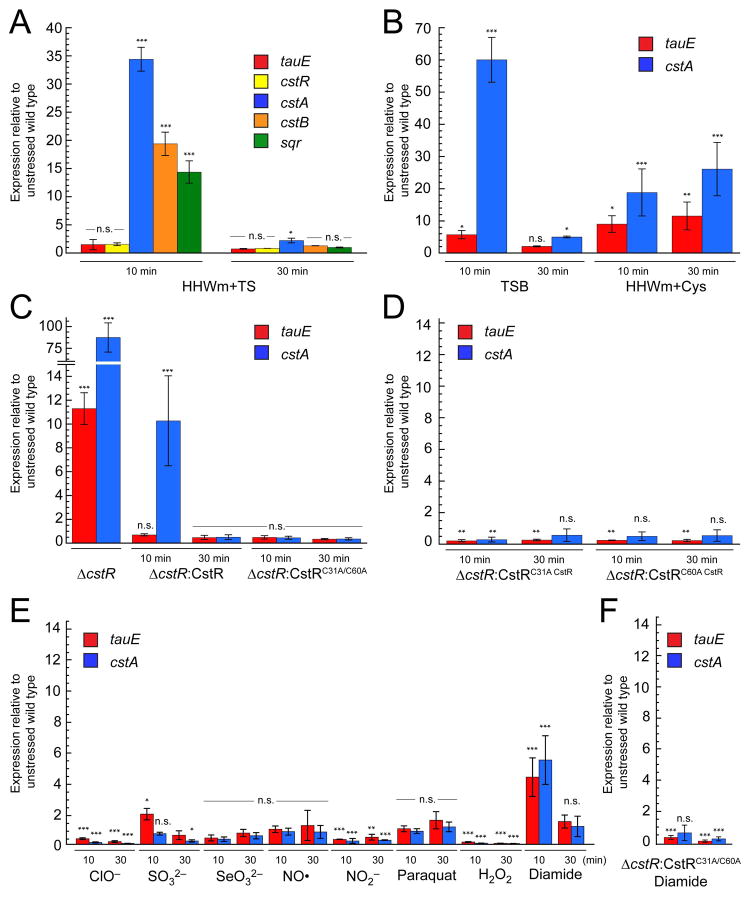
Figure 4. The cst operon is regulated by hydrogen sulfide in vivo.
Quantitative RT-PCR experiments for WT and mutant S. aureus cultures grown to an OD600 of 0.2 and challenged with 0.2 mM NaHS added to the growth medium at t=0. Aliquots for analysis were collected at 10 and 30 min post addition. All cultures were grown in HHWm+TS unless otherwise noted. (A) Relative expression levels for individual cst operon genes post addition of NaHS. (B) Levels of tauE and cstA expression in TSB (left) or HHWm+Cys (right). (C) ΔcstR (left), ΔcstR:CstR (middle), and ΔcstR:CstRC31A/C60A (right). (D) ΔcstR:CstRC31A (left) and ΔcstR:CstRC60A (right) individual cysteine mutants of CstR. (E) RT-PCR analysis for WT S. aureus exposed to acute toxicity of 2.4 mM hypochlorite (ClO−), 10 mM sulfite (SO32−), 0.2 mM selenite (SeO32−), 0.5 mM nitric oxide (NO) as MAHMA NONOate, 5 mM nitrite (NO2−), 25 nM paraquat, 10 mM hydrogen peroxide (H2O2), or 1 mM diamide. (F) ΔcstR:CstRC31A/C60A S. aureus exposed to 1 mM diamide stress. N = 3 error bars represent one s.d. from the mean, with fold-expression relative to wild-type, unstressed cells. Two-way ANOVA analysis was performed relative to 16S RNA at the indicated time point (*** = p < 0.001, ** = p < 0.005, * = p < 0.050, and n.s. = not statistically significant).