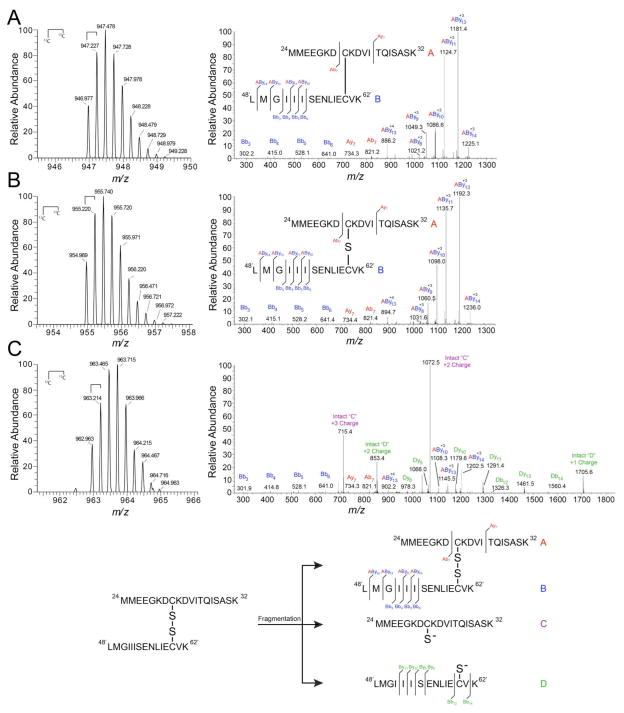
Figure 8. High-resolution tandem mass spectrometry confirms di-, tri-, and tetrasulfide mass shift assignments.
High-resolution LTQ-orbitrap tandem mass spectra of NaHS-reacted CstR tryptic peptides in the +4 charge state (left) and corresponding fragmentation patterns (right). Peptide A (red), 24MMEEGKDCKVITQISASK42, includes Cys31 and Peptide B (blue), 48′LMGIIISENLIECVK62′, includes Cys60’. Peptide fragments were assigned relative to either peptide “A” or “B” where Bb3 corresponds to the peptide fragment b ion 48′LMG50′ with a mass of 302 Da. Cross-linked peptides are denoted as “AByn” where peptide “A” remained intact and fragmentation occurred on peptide “B”. Inset: map of fragmentation pattern. (A) Disulfide. (B) Trisulfide. (C) Tetrasulfide.