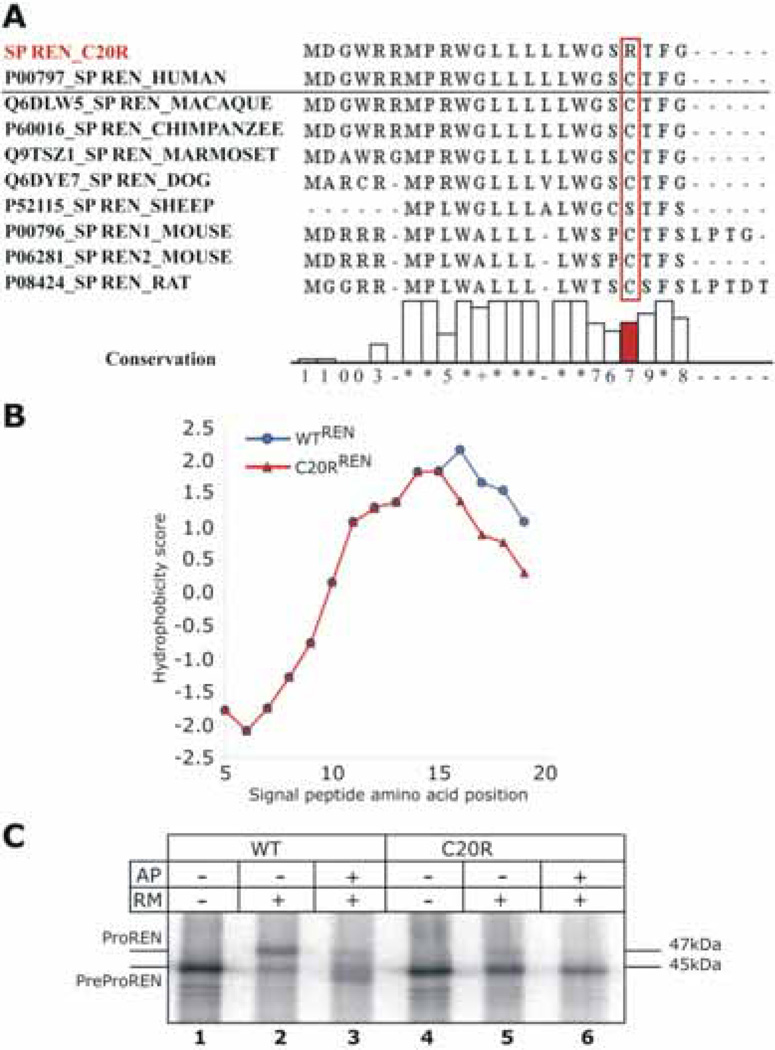
Figure 5.
Bioinformatic and in vitro characterization of mutant renin. A: Amino acid conservation of renin signal sequence across higher mammals. B: Hydrophobicity scores for the wild type and mutated preprorenin signal peptides. C: Nascent WTREN and C20RREN proteins translated in vitro from corresponding mRNAs in nuclease-treated rabbit reticulocyte lysate in the absence (–) or presence (+) of rough endoplasmic reticulum microsomes (RM) and tripeptide glycosylation acceptor (AP). Without RM and AP only nascent preprorenin (PreProREN) is formed (lanes 1 and 4). With RM and in the absence of AP most of the ER-translocated WTREN is converted into fully glycosylated prorenin (ProREN); (lane 2) whereas C20RREN produces only minute amounts of ProREN (lane 5). With RM and AP, the glycosylation of the ER-translocated WTREN is inhibited and partially glycosylated intermediates are present (lane 3); C20RREN, which is not ER-translocated, is present as non-glycosylated preprorenin.