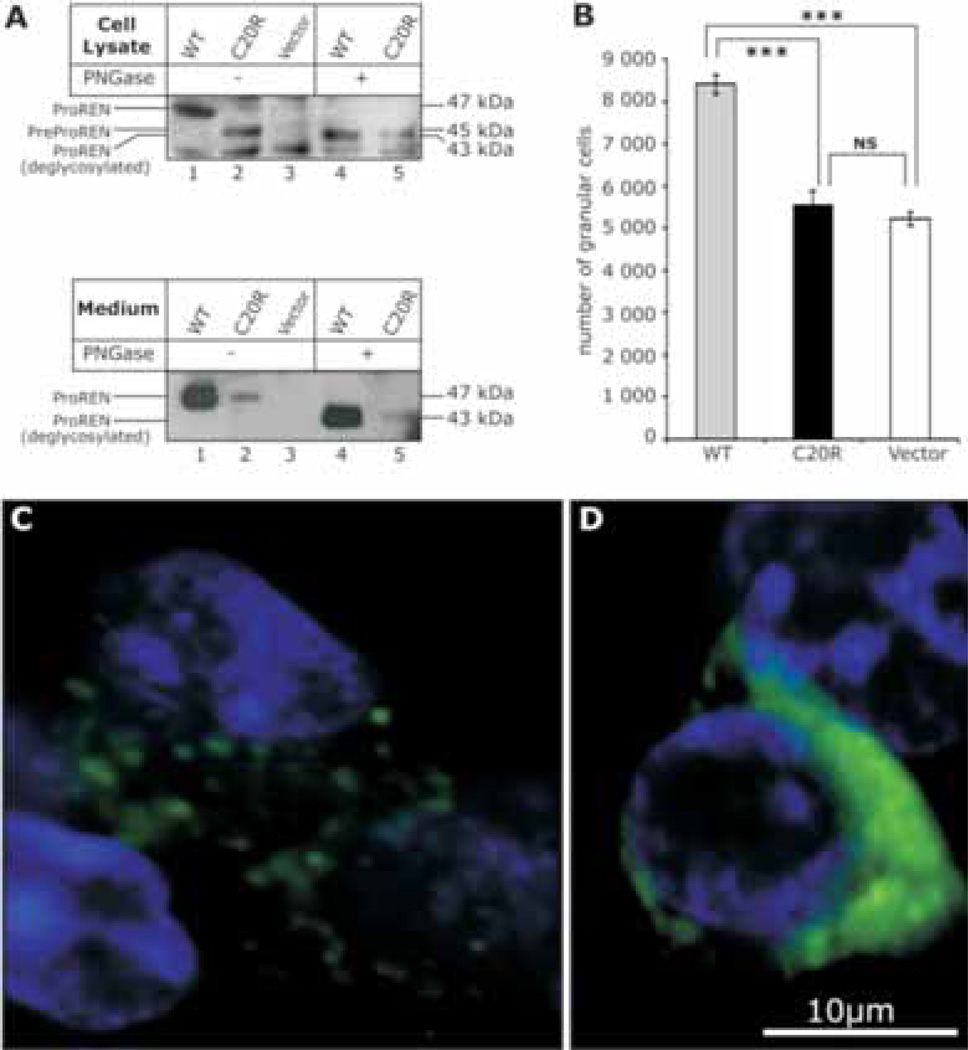
Figure 6.
Transient expression of WTREN and C20RREN preprorenin in HEK-293 cells. A: Western blot analysis of the protein products in cell lysates and medium showing that C20RREN produces only minute amounts of normally glycosylated, proteolytically processed and secretory competent prorenin (ProREN). To distinguish proteolytic processing and glycosylation status, the expressed proteins were analyzed before (–) and after (+) deglycosylation with PNGase. B: Fluorescence-activated cell sorter analysis showing inability of the C20RREN to form cytoplasmic granules. The values represent means ± s.d. of the three transfection experiments carried out in triplicates. The differences between the number of granular cells expressing the WTREN and cells expressing either the C20RREN or empty vector were statistically significant (p < 0,001). C,D: Cellular localization of preprorenin, prorenin and renin in HEK293 cells detected using antibodies recognizing amino acid residues 288 – 317 of the preprorenin (Yanaihara, Shizuoka, Japan). C: WTREN is present in a form of fine cytoplasmic granules. D C20RREN protein is retained intracellularly and shows intense diffuse cytoplasmic staining.