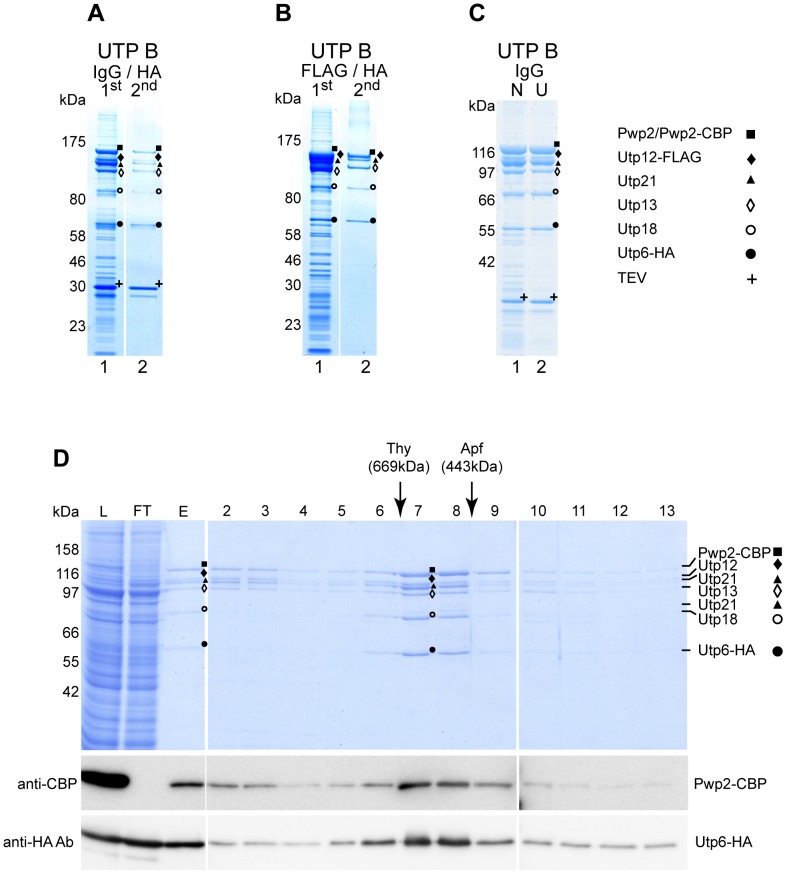
Figure 2. Yeast UTP B subcomplex reconstitution in insect cells.
All selected UTP B components were co-expressed in SF21 insect cells infected with baculoviruses containing the bacmids K1991 or K1992. The protein content of the indicated bands was identified by MS and are indicated as Pwp2, ▪; Utp6, •; Utp12, ♦; Utp13, ◊; Utp18, ○ and Utp21, ▴. (A) Lysates of 2×108 cells infected with K1991were used for two-step affinity purification. Pwp2-TAP was used as the bait protein in the first affinity purification step with IgG-coupled Sepharose resin, and Pwp2-containing components were eluted with TEV protease (Lane 1). Utp6-HA-containing components were purified from 90% of the first elution sample using anti-HA affinity matrix, followed by elution with the HA peptide (Lane 2). The composition of the eluate (5% each) was analyzed on a 4–12% gradient SDS-PAGE, stained with Coomassie Blue, and analyzed by MS. (B) Lysates of 2×108 cells infected with K1992 were used for two-step affinity purification. Utp12-FLAG was purified with anti-FLAG affinity matrix and eluted with the FLAG peptide during the first affinity purification step (Lane 1). A 90% aliquot of the eluted material was used to purify Utp6-HA-containing components with anti-HA affinity matrix, followed by elution with the HA peptide (Lane 2). The composition of both eluates (5%) was analyzed on a 4–12% gradient SDS-PAGE, stained with Coomassie Blue, and analyzed by MS. (C) Lysates of 8×107 SF21 cells infected with bacmid K1991 were cleared by the low-speed centrifugation described in the normal protocol (N samples), and half was further cleared by ultracentrifugation (200000×g, 1 h, 4°C, U samples). Pwp2-TAP-containing components were purified from both lysates using IgG-coupled Sepharose resin and eluted with TEV protease. A 10% aliquot of the eluted material was analyzed on a 4-12% gradient SDS-PAGE, stained with Coomassie Blue, and analyzed with MS. (D) Pwp2-TAP-containing components were purified from lysates of 4×107 infected cells (K1991) using IgG-coupled Sepharose resin and TEV elution. Half of the eluate was fractionated on a Superose 6 gel filtration column. Aliquots of the lysate (L, 0,03%), flow through from the first purification (FT, 0,03%), the eluate from the affinity column (E, 10%), and the fractions from the gel filtration column (2–13; 15%) were analyzed by SDS-PAGE (upper panel) and WB with antibodies against CBP (middle panel) or HA (lower panel) epitopes. Elution of marker proteins in independent gel filtration runs are indicated at the top. Correct identification by MS analysis of the corresponding protein is indicated.