Figure 3.
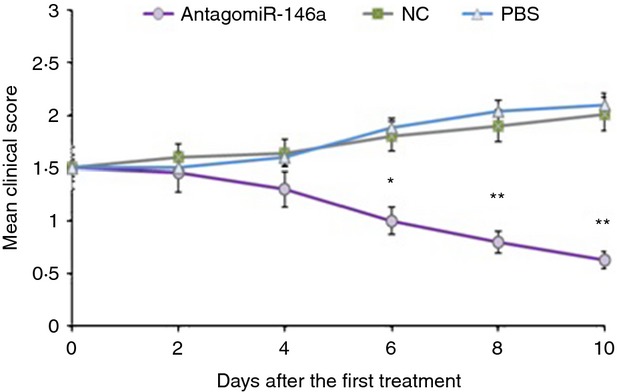
Treatment with AntagomiR-146a ameliorated clinical myasthenic symptoms in mice with ongoing experimental autoimmune myasthenia gravis (EAMG). Each symbol represents the mean clinical score (MCS) of mice in the AntagomiR-146a group (n = 10), the NC group (n = 10) and the PBS group (n = 10) at various times after treatment with respective treatment drugs via the caudal vein for 3 days continuously. Differences of the MCS were statistically significant between three groups since the sixth days after enrolment began. The MCS of AntagomiR-146a group was significantly lower than NC and PBS groups. At the end of the experiment, the MCS of the AntagomiR-146a group was 0·63 ± 0·33, the NC group was 2·01 ± 0·41, and the PBS group was 2·14 ± 0·55 (*P < 0·05; **P < 0·01).
