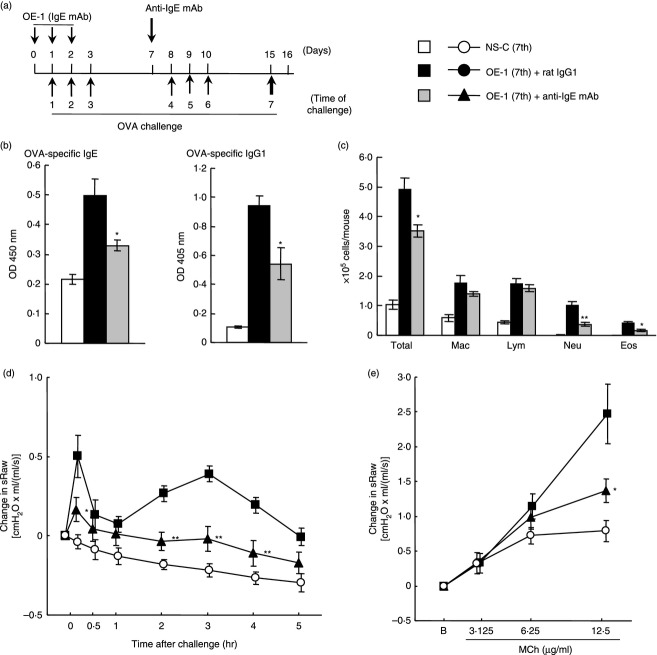
Figure 3.
Effect of anti-IgE monoclonal antibody (mAb) on IgE-mediated airway inflammation at the seventh challenge. (a) Experimental protocol for treatment with anti-IgE mAb. Anti-IgE mAb was intraperitoneally administered on day 7 [OE-1 (7th) + anti-IgE mAb]. Negative and positive controls were non-sensitized–challenged [NS-C (7th)] and OE-1-sensitized, rat IgG1 mAb-treated [OE-1 (7th) + rat IgG1] mice, respectively. (b) Effect of anti-IgE mAb on the increased levels of OVA-specific IgE and IgG1 in serum 24 hr after the seventh challenge (Day 16). (c) Effect of anti-IgE mAb on increase in the inflammatory cell numbers in BALF 24 hr after the seventh challenge. (d) Effect of anti-IgE mAb on the biphasic increase in airway resistance after the seventh challenge. (e) Effect of anti-IgE mAb on the development of AHR 24 hr after the seventh challenge. Each value is the mean ± SEM of four to seven animals from two separate experiments. *P < 0·05 and **P < 0·01 compared with OE-1 (7th) + rat IgG1 group.