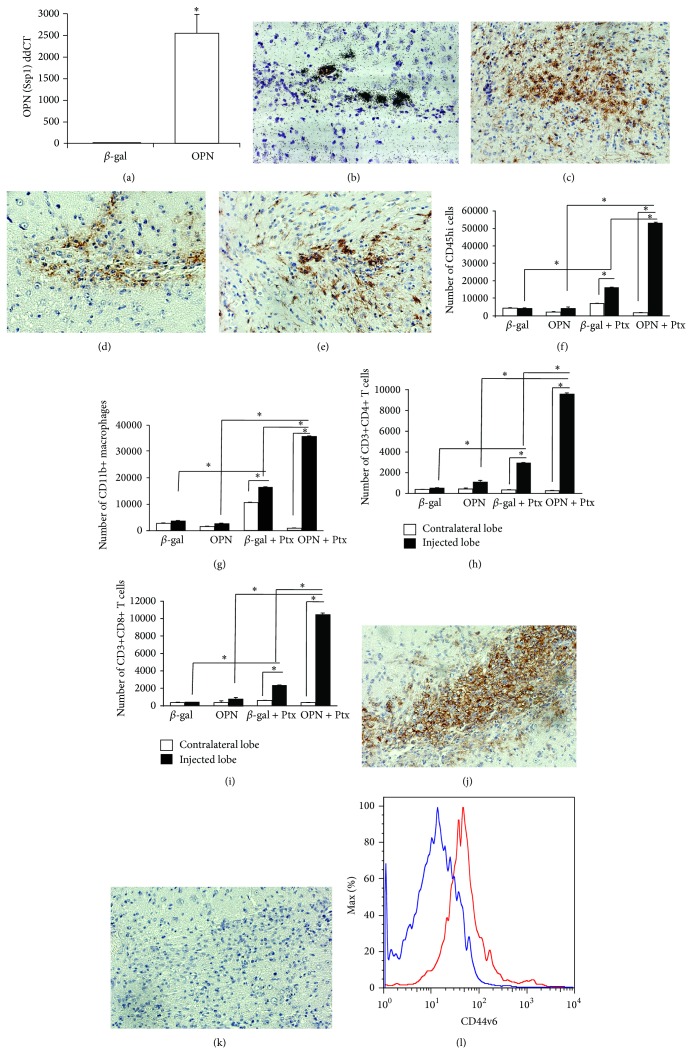
Figure 1.
Induction of OPN in the brain promotes migration of peripherally activated leukocytes. The adenoviral vector encoding the OPN gene, injected between the cortex and the putamen, induced OPN (Spp1) expression that was detected by (a) qRT-PCR for OPN (Ssp1) detection in lesion fragment site. * P < 0.05, t-test. (b) In situ hybridization for detection of OPN expressed in a representative lesion site tissue, 6 days after injection. Animals were also injected with pertussis toxin (Ptx) ip. The brains injected with the OPN-vector or with a control adenovirus encoding β-gal (β-gal), were separated in contralateral lobe (white bars) and injected lobe (black bars and transformed in cell suspensions as described [74]. (c) Iba-1+ cells (brown) with morphological characteristics of activated microglia in a site of lesion of a representative OPN-injected mice, showing that microglia activation was highly restricted to the delivery site, as detected by immunohistochemistry on a serial section. (d) F4/80+ cells (macrophages—brown) in the delivery site, 6 days after injection with OPN on a serial section of representative OPN-injected mouse; (e) Iba-1+ cells in β-gal-injected brains. The immune cell content, excluding resident microglia, was evaluated by FACS using fluorescent labeled antibodies against the indicated surface markers. (f) Gated CD45 high migrating cells, and among them (g) CD11b+ macrophages, (h) CD3+CD4+, and (i) CD3+CD8+ lymphocytes. * P < 0.05, Bonferroni's post hoc test. (j) F4/80+ cells (brown) in animals that received the OPN vector and were also stimulated with Ptx. (k) Isotype control staining on a Ptx-stimulated OPN-injected mouse. (l) Histogram of CD44v6 fluorescence intensity, on CD11b-gated cells, upon FACS analysis of brain-draining deep cervical lymph nodes, 6 days after i.p. injection of saline or Ptx into animals that received the OPN vector. Blue line shows animals injected with saline, and the red line shows cells from Ptx-stimulated mice.