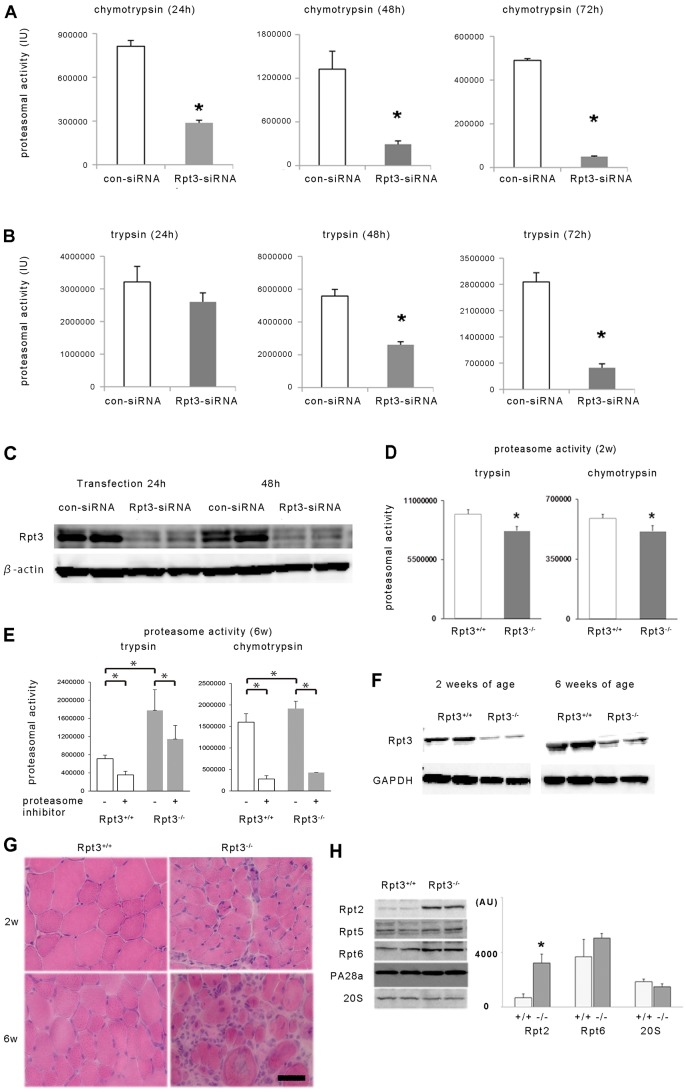
Fig. 4.
The effect of Rpt3 knockdown and Rpt3 deletion on proteasomal activity in vitro and in vivo. The chymotrypsin-like (A) and trypsin-like (B) activities of control (con-siRNA)- and Rpt3-siRNA-transfected C2C12 cells were assessed. Proteasomal activity was significantly suppressed in Rpt3-siRNA-transfected cells. Proteasomal activity at 24, 48 and 72 h after transfection is shown. IU, international units. (C) Rpt3 protein expression was suppressed in Rpt3-siRNA-transfected C2C12 cells. (D) Proteasomal activity (trypsin-like and chymotrypsin-like activity) in the tibialis anterior muscles of 2-week-old Rpt3−/− and Rpt3+/+ mice (n = 5). (E) Proteasomal activity (trypsin-like and chymotrypsin-like activity) in the tibialis anterior muscles of 6-week-old Rpt3−/− and Rpt3+/+ mice (n = 6). Trypsin-like and chymotrypsin-like activity were increased in Rpt3−/− mice compared with Rpt3+/+ mice. The highly specific proteasomal inhibitor AdaAhx3L3VS (30 µM) significantly suppressed chymotrypsin-like activity by >70%, and trypsin-like activity by >30% from tibialis anterior muscle homogenate of both 6-week-old Rpt3−/− and Rpt3+/+ mice. (F) Immunoblotting of Rpt3 protein using homogenates of tibialis anterior muscles from 2- and 6-week-old mice. (G) HE staining of tibialis anterior muscles from 2- and 6-week-old mice. Scale bar: 50 µm. (H) Changes in the components of the proteasomal complex of the tibialis anterior muscles. Rpt2 is significantly increased in Rpt3−/− mice compared with its expression in Rpt3+/+ mice. White bars, Rpt3+/+; gray bars, Rpt3−/−. AU, arbitrary units. All quantitative data show the mean+s.e.m.; *P<0.05 (Student's t-test).