Fig. 4. Lateral diffusion rate of CX3CL1-EYFP and its truncated, deglycosylated, or TM mutants.
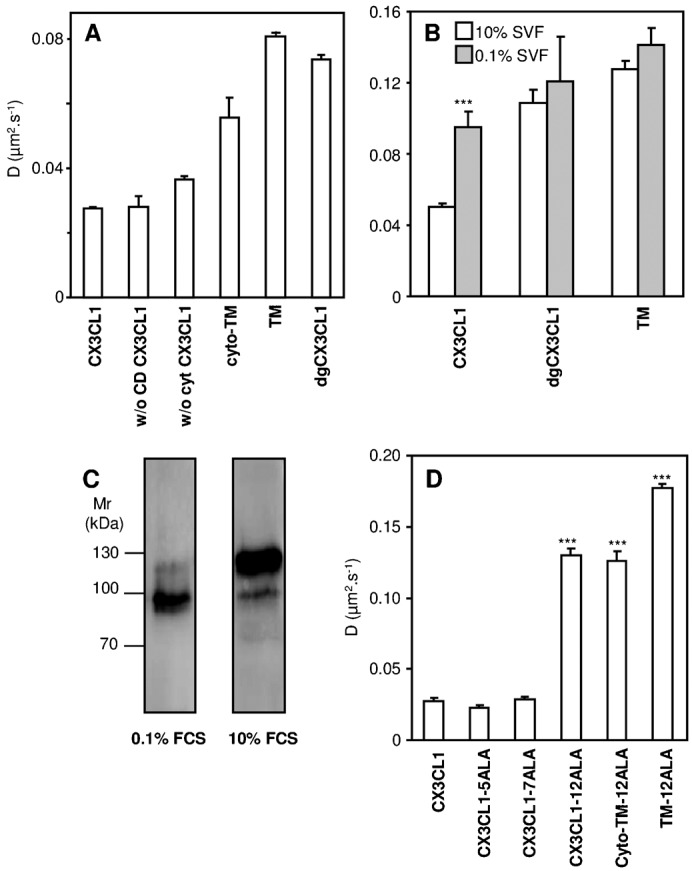
(A) Diffusion rate assessed by FRAP in COS7 cells of native CX3CL1-EYFP or CX3CL1-EYFP lacking its chemokine domain (named “w/o CD CX3CL1”), its cytosolic tail (named “w/o cyto CX3CL1”), its extracellular part (named “cyto-TM”), or both of its extracellular and intracellular regions (named “TM”) or deglycosylated (named “dgCX3CL1”); * p<0.05 and *** p<0.001 compared with native CX3CL1-EYFP. (B) Diffusion rate of CX3CL1-EYFP and various mutants as assessed by FRAP in CHOldlD cells cultured either with 10% FCS (empty bars) or 0.1% FCS (filled bars) medium. *** p<0.001 compared with the respective 10% FCS cultured CX3CL1-EYFP-expressing cells. (C) Immunoblotting of CX3CL1 chimera in CHOldlD. Thirty micrograms of membrane lysates from CHOldlD transfected with CX3CL1-EYFP and cultivated under normal (right) or deprived (left) serum conditions were loaded onto a 10% polyacrylamide gel under reducing conditions, transferred to a nitrocellulose membrane, and incubated overnight at 4°C under agitation with anti-human CX3CL1 primary antibody. The immune complexes were visualized with secondary peroxidase-conjugated antibodies using a chemiluminescent kit. (D) Lateral diffusion measured by FRAP in COS7 cells expressing the native CX3CL1-EYFP chimera or different constructions in which residues 321 to 325 were mutated to ALA (named “CX3CL1-5ALA”), residues 326 to 332 were mutated to ALA (“CX3CL1-7ALA”), or residues 321 to 332 were mutated to ALA (“CX3CL1-12ALA”). The lateral diffusion of CX3CL1-12ALA and of this mutated chemokine without its extracellular domain (“cyto-TM-12ALA”) or without its extra and intracellular domains (“TM-12ALA”) is also reported. *** p<0.001 compared with native CX3CL1-EYFP.
