Fig. 3. Binding of anti-mCherry-Darpin-GFP fusions to mCherry in HeLa cells.
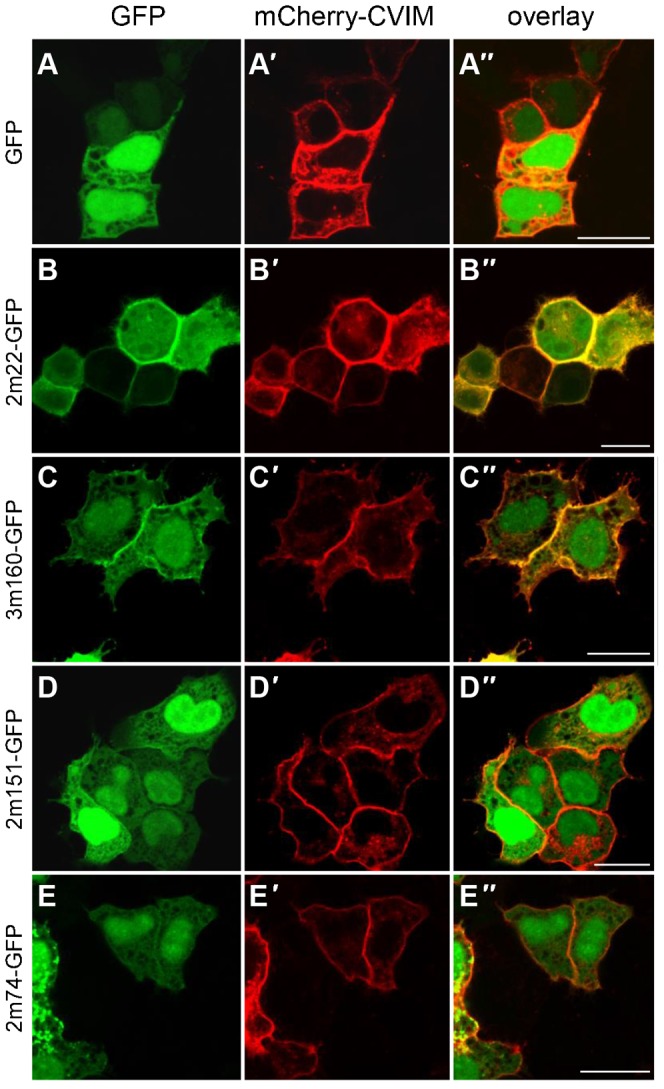
Shown are HeLa cells transiently overexpressing GFP (A) or anti-mCherry-GFP fusion proteins (B–E) together with a mCherry version tethered to the plasma membrane (mCherry-CVIM, A′–E′). Overlap of fluorescent signal indicates binding of the respective anti-mCherry-DARPin-GFP fusion to mCherry-CVIM, indicated by the yellow fluorescent signal at the plasma membrane. (A–A″) As expected, the untethered GFP control does not change its subcellular localization upon co-expression with mCherry-CVIM and remains cytoplasmic and nuclear. (B–B″) 2m22-GFP re-localizes to the plasma membrane where it binds to mCherry-CVIM. (C–C″) The 3m160-GFP fusion protein results in some fluorescent signal at the plasma membrane indicating the weaker affinity to mCherry-CVIM. (D–D″) 2m151-GFP and (E–E″) 2m74-GFP fusion proteins are not significantly recruited to the plasma because of their only micromolar affinity. Unprimed letters, GFP channel; primed letters, mCherry channel; double primed letters, overlay. Scale bars are 20 µm.
