Abstract
Progressive rising population of diabetes and related nephropathy, namely, diabetic kidney disease and associated end stage renal disease has become a major global public health issue. Results of observational studies indicate that most diabetic kidney disease progresses over decades; however, certain diabetes patients display a rapid decline in renal function, which may lead to renal failure within months. Although the definition of rapid renal function decline remained speculative, in general, it is defined by the decrease of estimated glomerular filtration rate (eGFR) in absolute rate of loss or percent change. Based on the Kidney Disease: Improving Global Outcomes 2012 clinical practice guidelines, a rapid decline in renal function is defined as a sustained decline in eGFR of > 5 mL/min per 1.73 m2 per year. It has been reported that potential factors contributing to a rapid decline in renal function include ethnic/genetic and demographic causes, smoking habits, increased glycated hemoglobin levels, obesity, albuminuria, anemia, low serum magnesium levels, high serum phosphate levels, vitamin D deficiency, elevated systolic blood pressure, pulse pressure, brachial-ankle pulse wave velocity values, retinopathy, and cardiac autonomic neuropathy. This article reviews current literatures in this area and provides insight on the early detection of diabetic subjects who are at risk of a rapid decline in renal function in order to develop a more aggressive approach to renal and cardiovascular protection.
Keywords: Type 2 diabetes, Diabetic kidney disease, Rapid decline, Estimated glomerular filtration rate, Albuminuria
Core tip: The progression rate of diabetic kidney disease is highly variable, a rapid decline of renal function can lead to renal failure within months. Risk factors account for rapid decline renal function in patients with type 2 diabetes include ethnic/genetic and demographic factors, lifestyle and health behaviors, advanced albuminuria, poor glycemic control, dyslipidemia and some biochemical abnormalities. Diabetic patients with retinopathy or cardiac autonomic neuropathy are at increased risk of a rapid decline in estimated glomerular filtration rate. Early detection of high-risk groups with a more aggressive multifactorial approach to renal and cardiovascular protection is important.
INTRODUCTION
Type 2 diabetes is one of the leading causes of chronic kidney disease (CKD) worldwide, and diabetic kidney disease has become a major global public health issue[1]. Early detection and intervention in diabetic kidney disease can help to slow renal function decline, prevent complications, and decrease cardiovascular events, thereby improving survival and quality of life in type 2 diabetics[2]. However, potential causes accounting for variation in diabetic kidney disease and its rate of progression are still largely unexplored. In most cases, disease progresses over decades; however, a rapid decline in renal function can lead to renal failure within months[3]. Thus, in type 2 diabetics, defining high-risk groups and preventing or retarding disease progression is an emerging challenge. This review targets the potential risk factors of a rapid decline in renal function in patients with type 2 diabetes.
EPIDEMIOLOGY OF DIABETIC KIDNEY DISEASE
Diabetic kidney disease is identified clinically through the presence of albuminuria, impaired glomerular filtration rate (GFR), or both[4], and these two biomarkers have been used for the diagnosis, severity classification, and outcome prediction of CKD[5-8]. The categories of albuminuria are defined as microalbuminuria or macroalbuminuria based on a urinary albumin-to-creatinine ratio (UACR) of 30-300 mg/g, or > 300 mg/g, respectively[9,10], and impaired renal function is defined as an estimated glomerular filtration rate (eGFR) < 60 mL/min per 1.73 m2[1,4,10]. International consensus on the incidence of CKD in patients with type 2 diabetes is lacking[11]. Although the prevalence of diabetic CKD is increasing worldwide, there are large differences between regions and ethnicities (Table 1). A report from the UK Prospective Diabetes Study (UKPDS), states that 1544 (38%) of 4031 patients developed albuminuria (microalbuminuria or macroalbuminuria), and 1449 (29%) of 5,032 patients developed renal impairment (based on the Cockroft-Gault formula of eGFR < 60 mL/min per 1.73 m2) over a 15-year period[12]. Meanwhile, the Developing Education on Microalbuminuria for Awareness of renal and cardiovascular risk in Diabetes (DEMAND) study, in which data from 32208 type 2 diabetics from 33 countries were collected, reported that overall global prevalence of microalbuminuria and macroalbuminuria was 39% and 10% respectively, while eGFR below 60 mL/min per 1.73 m2 occurred in 22% of the 11573 patients with available data[13]. According to the US Renal Data System (USRDS) 2013 report, 3 out of 5 new end stage renal disease (ESRD) patients came from diabetes in Malaysia, Mexico, and Singapore; furthermore in the United States, the odds ratios of diabetes in albuminuria (UACR more than 30 mg/g) and CKD (defined as eGFR below 60 mL/min per 1.73 m2) were 3.9 and 2.1 respectively[14]. It was recently reported that 30% of CKD in 5584 Chinese patients aged 20-79 years, was associated with dysglycemia (diabetes and prediabetes), independent of age, sex, and hypertension status[15]. It should be noted that some limitations and pitfalls were identified in these epidemiological data, for example, demographic distribution[11], socioeconomic status[16], dynamic changes in the incidence of diabetes, changes in the use of medication (including anti-diabetic drugs and anti-hypertensive drugs), and the improvement of survival rates in diabetic and ESRD patients[11].
Table 1.
Prevalence of albuminuria and impaired glomerular filtration rate in diabetic patients
| Ref. | Population (Nationality) | Albuminuria prevalence | Impaired GFR prevalence |
| Parving et al[13] | International DEMAND study of 33 countries 2006 32208 type 2 diabetic patients | Microalbuminuria: 39% Macroalbuminuria: 10% | 22% |
| Bos et al[108] data from: Herman et al[109] Hamed et al[111] | Northern Africa Systematic review of PubMed 1990-2012 > 18 years old diabetic patients | Egypt 1998: Albuminuria: 21%[109] Sudan 2008 (insulin treated diabetic patients): Albuminuria: 22%[110] | Egypt 1998-Outpatient clinics: 6.7%[109] Egypt 1995-Hospital inpatients: 46.3%[111] |
| Icks A and Koch M Epidemiology of chronic kidney disease in diseases. In: Wolf G. Diabetes and Kidney Disease[11], data from: Chadban et al[112] | Australia AusDiab study: a national population-based cross-sectional survey > 25 years old diabetic patients | 8.70% proteinuria-spot urine protein to creatinine ratio (abnormal: > 0.20 mg/mg) | 27.60% |
| Unnikrishnan et al[113] | Southern India CURES 45 study 17, 16 type 2 diabetic patients | Microalbuminuria: 36.9% Macroalbuminuria: 2.2% | - |
| Icks A and Koch M Epidemiology of chronic kidney disease in diseases. In: Wolf G. Diabetes and Kidney Disease[11], data from: Lin et al[114] | Taiwan Community-based screening 1999–2001 > 30 years old type 2 diabetic patients | 29.40% proteinuria-spot urine protein to creatinine ratio (abnormal: > 0.20 mg/mg) | 15.10% |
| Yang et al[115] | China A nationally representative sample from 14 provinces and municipalities > 20 years old diabetic patients | 17.30% | 19.10% |
| Lou Arnal et al[116] | Spain A survey of 16 Health Centers of the Alcañiz Health Sector 2008 > 18 years old, 3466 type 2 diabetic patients | 31.70% | 25.20% |
| Detournay et al[117] | France ENTRED data 2007 A survey of the national public prescription claims database Type 2 diabetic patients | - | 22% |
| Collins et al[14] | United Status NHANES study 2005-2010 Adult diabetic patients | 29.90% | 19.30% |
| Al-Rubeaan et al[54] | Saudi Arabia SNDR data > 25 yr, 54670 type 2 diabetic patients | Microalbuminuria: 1.2% Macroalbuminuria: 8.1% | GFR < 30 mL/min per 1.73 m2: 1.50% |
Albuminuria: Albumin-to-creatinine ratio (UACR) > 30 mg/g; Microalbuminuria: UACR 30-300 mg/g; Macroalbuminuria: UACR > 300 mg/g; Impaired glomerular filtration rate (GFR): Estimated GFR < 60 mL/min per 1.73 m2; DEMAND: Developing Education on Microalbuminuria for Awareness of renal and cardiovascular risk in Diabetes study; AusDiab: The Australian Diabetes, Obesity and Lifestyle Study; CURES: Chennai Urban Rural Epidemiology Study; ENTRED: Échantillon national témoin représentatif des personnes diabétiques (National Representative Sample of Diabetic Patients); NHANES: National Health and Nutrition Examination Survey; SNDR: Saudi National Diabetes Registry.
DEFINING A RAPID DECLINE IN RENAL FUNCTION
Annual decline in GFR in an individual varies widely depending on race, age, the presence of underlying conditions, the etiology of CKD, and the presence of comorbidities. A previous study reported that age-related eGFR decline is about 0.75-1 mL/min per 1.73 m2 per year over 40 years of age[17]. Among the healthy population, eGFR decline is approximately 0.36-1.21 mL/min per 1.73 m2 per year[5,18-21]. A community-based cohort study reported a decline in eGFR of 2.1 and 2.7 mL/min per 1.73 m2 per year respectively for women and men with diabetes, whereas the rate of decline was 0.8 and 1.4 mL/min per 1.73 m2 per year respectively for women and men without diabetes[18,22]. In subjects with CKD, a more rapid decline in renal function (ranging 1.03-4.3 mL/min per 1.73 m2 per year) was noted[10,23-26] (Table 2). Some studies define rapid decline of eGFR in terms of absolute rate of loss, while others define it as percent change (Table 3)[3,27-30]. According to the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 clinical practice guidelines for the evaluation and management of CKD, developed by the National Kidney Foundation, a rapid decline in renal function is defined as a sustained decline in eGFR of > 5 mL/min per 1.73 m2 per year (as estimated using the 2009 CKD-EPI creatinine equation)[31]. It is generally believed that at present, there are a lack of well-controlled studies, which include frequent measurements and a long follow-up period, from which to establish an optimal definition of a rapid decline in renal function[18].
Table 2.
Decline of estimated glomerular filtration rate in different populations
| Population | eGFR decline (mL/min per 1.73 m2 per year) | Ref. |
| Healthy | ||
| PREVEND study 6894 subjects | 0.55 | Halbesma et al[5] |
| Estimated using MDRD formula | ||
| Annual health exam, Japan | 0.36 | Imai et al[19] |
| 120727 subjects | Estimated using MDRD formula modified by a Japanese coefficient | |
| ARIC study | 0.47 | Matsushita et al[20] |
| 13029 subjects | Estimated using MDRD formula | |
| Tromso Study, Norway | 1.21 (men) | Kronborg et al[21] |
| 2249 men and 2192 women | 1.19 (women) Estimated using MDRD formula | |
| Aged without diabetes | ||
| 2475 men > 65 years old | 1.4 | Hemmelgarn et al[22] |
| 3163 women > 65 years old | 0.8 | Hemmelgarn et al[22] |
| Aged with diabetes | ||
| 490 men > 65 years old | 2.7 | Hemmelgarn et al[22] |
| 445 women > 65 years old | 2.1 | Hemmelgarn et al[22] |
| CKD | ||
| MDRD study group | 3.7 | MDRD study group |
| eGFR 25-80 mL/min per 1.73 m2, n = 28 | Levey et al[23] | |
| eGFR 7.5-24 mL/min per 1.73 m2, n = 63 | 4.3 | |
| African Americans with hypertension eGFR 20-65 mL/min per 1.73 m2 low mean arterial pressure, n = 380 | 2.21 | Wright et al[24] |
| normal mean arterial pressure, n = 374 | 1.95 | |
| Tromso Study, Norway | 1.03 | Eriksen et al[25] |
| eGFR 30-59 mL/min per 1.73 m2 3047 subjects | ||
| eGFR < 60 mL/min per 1.73 m2 4231 subjects | 2.65 | Levin et al[26] |
Data from Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group[18]; eGFR: Estimated glomerular filtration rate; CKD: Chronic kidney disease; PREVEND: Prevention of Renal and Vascular End-Stage Disease; ARIC: Atherosclerosis Risk in Communities; MDRD: Modification of Diet in Renal Disease Study.
Table 3.
Definitions of rapid renal function decline
| Population (Nationality) | Rapid renal function decline | Ref. | |
| Study | |||
| United States 4380 patients from the community-based CHS ≥ 65 years old Follow-up: 7 yr 14% with diabetes | > 3 mL/min per 1.73 m2 per year | Reviewed by KDIGO CKD Work Group[18]: Shlipak et al[28] Rifkin et al[30] | |
| Taiwan 577 type 2 diabetes patients from an outpatient department in a hospital-based study 63 years old (mean age) Follow-up: 1 yr | > 3 mL/min per 1.73 m2 per year | Sheen et al[72] | |
| 472 CKD 4-5 patients from an outpatient department in a hospital-based study 65 years old (mean age) 35.4% with diabetes Follow-up: 1.5 yr (17.3 mo) | Tsai et al[118] | ||
| Canada 4231 patients with eGFR < 30 mL/min per 1.73 m2 from a cohort derived from all patients registered in a provincial database Follow-up: 2.5 yr (31 mo) | > 4 mL/min per 1.73 m2 per year | Levin et al[26] | |
| Italy 1682 type 2 diabetes patients with eGFR ≥ 60 mL/min per 1.73 m2 from an outpatient department in a hospital based study Follow-up: 10 yr | > 4% per year | Zoppini et al[70] | |
| Canada 3154 patients with eGFR ≥ 60 mL/min per 1.73 m2, from the community based Walkerton Health Study (2002 to 2008) Follow-up: 7 yr | > 5% per year | Clark et al[74,119] | |
| Taiwan 7968 civil servants and teachers ≥ 50 years old (mean age: 57 years old) Follow-up: 15 yr | > 20% per year | Reviewed by KDIGO CKD Work Group[18]: Cheng et al[29] | |
| Taiwan 167 patients in a hospital based study | > 25% per year | Chen et al[85] | |
| Review | Chronic kidney disease Lancet | > 4 mL/min per 1.73 m2 per year | Levey et al[3] |
| Guideline | KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease KDIGO CKD Work Group | > 5 mL/min per 1.73 m2 per year | Inker et al[10] KDIGO CKD Work Group[18] |
CHS: Cardiovascular Health Study; KDIGO: Kidney Disease: Improving Global Outcomes; eGFR: Estimated glomerular filtration rate; CKD: Chronic kidney disease.
RISK FACTORS OF A RAPID DECLINE IN RENAL FUNCTION
An emerging challenge is the identification of potential factors associated with rapid renal function decline, which would form the basis for the development of strategies to prevent or retard disease progression, and reduce complications, thereby improving disease outcomes and quality of life in type 2 diabetics. Potential risk factors include ethnic/genetic and demographic factors, lifestyle and health behaviors, metabolic and biochemical abnormalities, cardiovascular functional factors, and some clinical symptoms of type 2 diabetes (Figure 1).
Figure 1.
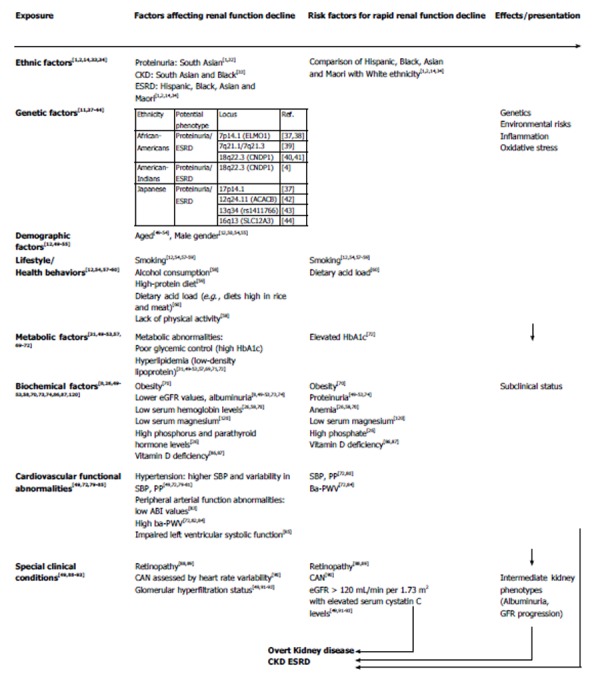
Conceptual model for diabetic kidney disease and potential risk factors of rapid renal function decline. CKD: Chronic kidney disease; HbA1c: Glycated hemoglobin; ESRD: End stage renal disease; eGFR: Estimated glomerular filtration rate; SBP: Systolic blood pressure; ba-PWV: Brachial-ankle pulse-wave velocity; PP: Pulse pressure; CAN: Cardiac autonomic neuropathy; ELMO1: Engulfment and cell motility 1; CNDP1: Carnosine dipeptidase 1; ACACA: Acetyl-CoA carboxylase alpha; rs: RefSNP (Single Nucleotide Polymorphism) numbers; SLC: Solute-carrier group.
Ethnic, genetic, and demographic factors
Ethnicity is a one of major factors affecting the progression of CKD in diabetic patients. In the United Kingdom, residents of South Asian origin had a higher prevalence of overt proteinuria and a lower prevalence of microalbuminuria compared to those with White European ethnicity[1,32]. In a 5-year retrospective, community-based cohort study of 135 general practices in East London, in which 3855 diabetic patients with an eGFR of < 60 mL/min per 1.73 m2 were enrolled, renal function decline occurred at a significantly higher rate in South Asians as compared to other ethnicities[33]. According to the USRDS 2012 annual data report[34], ESRD caused by diabetes has increased in African-American, Native American, and Hispanic populations over the past decade[1,2,34]. USRDS 2013 also reported that the contribution of diabetes to ESRD was 59%-61% in Malaysia, Mexico, and Singapore in 2011, and above 40% in Israel, the Republic of Korea, Hong Kong, Taiwan, the Philippines, Japan, the United States, and New Zealand[14]. In summary, diabetic patients of Hispanic, black, Asian, and Maori ethnicity are at a higher risk of a rapid decline in renal function compared to white populations.
Ethnic differences in the presentation of diabetic kidney disease may reflect either genetic predisposition or differences in public health care policy[1], and thus, genetic studies need to exclude non-genetic confounders. Evidence of genes associated with diabetic nephropathy in type 2 diabetics comes mainly from family-based genome-wide linkage studies[35,36]. Findings from such studies include reports that 7p14.1 [engulfment and cell motility 1 (ELMO1)][37,38], 7q21.1/7q21.3[39] and 18q22.3 [carnosine dipeptidase 1 (CNDP1)][40,41] are associated with the development of proteinuria and ESRD in African-Americans; 18q22.3 (CNDP1) is associated with proteinuria and ESRD in American-Indians[4]; and 17p14.1[37], 12q24.11 [acetyl-CoA carboxylase alpha (ACACB)][42], 13q34(rs1411766)[43], and 16q13 [solute-carrier group (SLC12A3)][44] may be associated with proteinuria and ESRD in Japanese[36]. Furthermore, haptoglobin (Hp) is a hemoglobin-binding protein that has a major role in protecting against heme-driven oxidative stress. Previous studies have shown the importance of the Hp genotype in the progression of diabetic nephropathy[45,46]. Moreover, diabetic patients with Hp 2-2 are more likely to develop nephropathy than those with Hp2-1 or Hp1-1[47,48].
Demographic factors may also influence the progression of diabetic kidney disease. Previous studies indicate that age is a significant predictor of progressive albuminuria and renal dysfunction in diabetics[49-54], and most studies reported that male sex is an important independent factor associated with renal function decline in type 2 diabetics[12,50,54,55]; however, some studies have shown an association with female sex[56].
Lifestyle and health behaviors
Smoking is an established factor for increased risk of development and rapid progression of diabetic kidney disease[12,54,57-59]. Also, some studies suggest an association between diet and renal function decline in diabetics, for example in those with high alcohol consumption[58] or a high-protein diet[59]. It has been demonstrated that a high dietary acid load (e.g., in diets high in rice and meat) is associated with rapid progression of diabetic nephropathy to ESRD in Westernized South Asian people[60]. Lack of physical activity is also considered to be a risk factor in diabetic nephropathy[58], with a previous study reporting that high physical activity in women was associated with an improvement in eGFR[21].
Metabolic and biochemical factors
A number of metabolic conditions, such as hyperglycemia[61,62], dyslipidemia[63-65], or being overweight/obese[31,49,66], are widely recognized as being associated with the development of diabetic nephropathy, and are established factors in identifying subjects at a greater risk of disease progression[57]. Previous studies indicate that obesity, hyperglycemia, and dyslipidemia are significant predictors of progressive albuminuria[49-53,67,68]. A recent cross-sectional study reported UACR significantly correlated with metabolic syndrome and its components, including hyperglycemia, central obesity, and high triglyceride levels[65,69]. Factors associated with eGFR decline and progressive albuminuria might overlap. During a 10-year follow-up, an observational study of 1682 type 2 diabetics with baseline eGFR ≥ 60 mL/min per 1.73 m2 reported that obese patients had a significantly faster age-adjusted annual eGFR decline[70]. A positive association between glycated hemoglobin (HbA1c) and CKD has also been observed in type 2 diabetics, even in the absence of albuminuria and retinopathy[52]. An association between blood glucose, low-density lipoprotein abnormalities, and the progression of renal damage in diabetes has been reported[71]. HbA1c was found to be independently associated with rapid renal function decline in a group of type 2 diabetics without symptomatic cardiovascular disease[72].
Albuminuria and eGFR are not only biomarkers for the diagnosis and categorization of CKD[4], but are also well-known predictors of renal function decline, ESRD, and death in type 2 diabetics[8,73]. Proteinuria is associated with rapid decline in renal function[49-53], and a previous study suggests that dipstick proteinuria measurement could be used as a screening tool for rapid renal function decline[74].
Abnormalities in cardiovascular function
CKD shares many risk factors with cardiovascular disease[72,75], and dysfunction in one system can often lead to dysfunction in the other[49]. In patients with concomitant hypertension and type 2 diabetes, the risk of progression to ESRD is 7 fold that for age-matched control subjects[49,76]. Hypertension is a significant risk factor for insufficient renal function, cardiovascular events, and death in patients both with and without type 2 diabetes[49,61,77-79]. Previous studies show that systolic blood pressure (SBP) and pulse pressure are stronger predictors than diastolic blood pressure of renal outcomes, and are independent risk factors in the rapid decline of eGFR in type 2 diabetics[72,80], while another study suggests that both SBP and variability in SBP are risk factors in the development and progression of diabetic nephropathy[81].
In addition to blood pressure, peripheral arterial functional markers are also associated with renal function in type 2 diabetics[82]. A low ankle-brachial index was found to be significantly associated with a low eGFR[83]. Also, arterial stiffness is associated with incident albuminuria and decreased eGFR[72,84], and brachial-ankle pulse-wave velocity (ba-PWV) values are independently associated with rapid renal function decline in type 2 diabetics without symptomatic cardiovascular disease[72]. One study reports that impaired left ventricular systolic function and increased ba-PWV are independently associated with a rapid decline in renal function[85].
Miscellaneous
Some other factors, such as low hemoglobin levels and electrolyte imbalance, may cause a rapid progression in diabetic kidney disease. Conditions including anemia, low serum magnesium levels, and high phosphorous and parathyroid hormone levels, are associated with rapid renal function decline in type 2 diabetics[26,58,70]. Furthermore, vitamin D deficiency associated with albuminuria was an independent risk factor in diabetic nephropathy after adjusting for demographic factors, hypertension, dyslipidemia, smoking status, and medication use[86,87].
Type 2 diabetic patients with additional microvascular complications, such as retinopathy or neuropathy, may also experience a rapid decline in renal function. Several studies have demonstrated that the rate of renal disease progression in type 2 diabetics with retinopathy is faster than that observed in those without retinopathy[88,89]; thus, screening for retinopathy may be helpful in identifying high-risk patients. Another study on cardiac autonomic neuropathy that assessed heart rate variability suggests that this is also an independent predictor of eGFR decline and could also be used as an identifying factor[90].
Special issues
Glomerular hyperfiltration and rapid renal function decline in type 2 diabetes: A longitudinal study of 600 type 2 diabetics with albuminuria < 200 μg/min, found that those with an eGFR > 120 mL/min per 1.73 m2 had a higher risk of albuminuria progression (hazard ratio: 2.16) compared with those without baseline hyperfiltration; over a 4-year follow-up, renal function decline was relatively rapid, at an annual rate of up to 3.37 mL/min per 1.73 m2[91]. Another study evaluated type 2 diabetic Pima Indians selected from participants in the Diabetic Renal Disease Study, with a baseline iothalamate clearance above the median for the entire study cohort (120 mL/min per 1.73 m2) to give a study group with a normal or elevated GFR[92]. After a mean follow-up of 3.8 years, it was shown that directly measured GFR declined at 4.4% per year, and supposed that an increase in serum cystatin C provide means for detecting early renal function decline in diabetes[92]. Measurement of serum cystatin C may help to identify groups at high risk of renal function decline based on hyperfiltration status[49,93].
Non-albuminuric diabetic kidney disease: Renal insufficiency in the absence of albuminuria in patients with type 2 diabetes is another issue that should be noted. In a 1977 study of type 2 diabetic adults, 13% had an eGFR < 60 mL/min per 1.73 m2, and 30% had neither albuminuria nor retinopathy[94]. Furthermore, data from UKPDS[12], DEMAND[13], and Atherosclerosis risk in Communities (ARIC)[52] studies suggests that the occurrence of renal impairment in type 2 diabetics without albuminuria is not unusual[49]. Microalbuminuria and reduced eGFR have been suggested as markers of different pathologic processes, with microalbuminuria associated with endothelial dysfunction and reduced eGFR being a renal manifestation of systemic atherosclerosis[49,95]. These patients are at higher risk of CKD progression, as the absence of proteinuria may lead to delays in the diagnosis and treatment of diabetic nephropathy[1,49].
POSSIBLE MANAGEMENT STRATEGIES
A number of therapeutic interventions for diabetic kidney disease have been developed over the past few decades[96]. Several studies have demonstrated increased activity in the renin-angiotensin-aldosterone system in diabetic patients with nephropathy[97,98]. Angiotensin-converting enzyme inhibitor (ACEI) and angiotensin receptor blocker (ARB) treatment for diabetics with hypertension can reduce renal damage and may reduce cardiovascular complications[97-99]; thus, ACEI or ARB are recommended as a first-line treatment for diabetics with hypertension[2,10,98,100,101]. However, based on the ONTARGET trial, acute dialysis, hyperkalemia, and hypotension tended to be more frequent with the use of both ACEI and ARB; thus, dual inhibition of the renin-angiotensin system is not recommended[102]. Primary multifactorial interventions aimed at slowing progression of diabetic nephropathy include combination therapy targeting hyperglycemia, hypertension, microalbuminuria, and dyslipidemia[59]. The Steno-2 study, of 151 type 2 diabetics with baseline microalbuminuria who underwent multifactorial treatment, reported that at a 7.8-year follow-up 46 patients showed remission to normoalbuminuria, improved hypertensive and glycaemic control were independent predictors for remission, and that kidney function may have been preserved through a slower rate of eGFR decline[103]. Other studies provide evidence that intensive multifactorial management is more effective than conventional treatment[104-107]. In addition to blood pressure, glycemic and lipid control, lifestyle modifications such as cessation of smoking, protein restriction in diets, weight reduction[2,59], light to moderate exercise[4], and vitamin C[104,105] and vitamin D supplementation[26], may be helpful in preventing or slowing the progression of diabetic kidney disease[2,26,59].
CONCLUSION
The progression of diabetic kidney disease is highly variable. According to the KDIGO 2012 clinical practice guidelines for the evaluation and management of CKD, a rapid decline in renal function was defined as a sustained decline in eGFR of > 5 mL/min per 1.73 m2 per year. Associated risk factors in patients with type 2 diabetes include ethnic/genetic and demographic factors, lifestyle and health behaviors, advanced albuminuria, poor glycemic control, dyslipidemia, and some biochemical abnormalities. Diabetic patients with retinopathy or cardiac autonomic neuropathy are at increased risk of a rapid decline in eGFR. Furthermore, those with glomerular hyperfiltration and elevated serum cystatin C may also be at increased risk of a rapid decline in renal function. Early detection of high-risk groups with a more aggressive multifactorial approach to renal and cardiovascular protection is important.
Footnotes
P- Reviewer: Nakhoul FM, Tamemoto H S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
References
- 1.Reutens AT. Epidemiology of diabetic kidney disease. Med Clin North Am. 2013;97:1–18. doi: 10.1016/j.mcna.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Stanton RC. Clinical challenges in diagnosis and management of diabetic kidney disease. Am J Kidney Dis. 2014;63:S3–21. doi: 10.1053/j.ajkd.2013.10.050. [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379:165–180. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- 4.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532–2539. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halbesma N, Kuiken DS, Brantsma AH, Bakker SJ, Wetzels JF, De Zeeuw D, De Jong PE, Gansevoort RT. Macroalbuminuria is a better risk marker than low estimated GFR to identify individuals at risk for accelerated GFR loss in population screening. J Am Soc Nephrol. 2006;17:2582–2590. doi: 10.1681/ASN.2005121352. [DOI] [PubMed] [Google Scholar]
- 6.Stephen R, Jolly SE, Nally JV, Navaneethan SD. Albuminuria: when urine predicts kidney and cardiovascular disease. Cleve Clin J Med. 2014;81:41–50. doi: 10.3949/ccjm.81a.13040. [DOI] [PubMed] [Google Scholar]
- 7.Holtkamp FA, de Zeeuw D, de Graeff PA, Laverman GD, Berl T, Remuzzi G, Packham D, Lewis JB, Parving HH, Lambers Heerspink HJ. Albuminuria and blood pressure, independent targets for cardioprotective therapy in patients with diabetes and nephropathy: a post hoc analysis of the combined RENAAL and IDNT trials. Eur Heart J. 2011;32:1493–1499. doi: 10.1093/eurheartj/ehr017. [DOI] [PubMed] [Google Scholar]
- 8.Berhane AM, Weil EJ, Knowler WC, Nelson RG, Hanson RL. Albuminuria and estimated glomerular filtration rate as predictors of diabetic end-stage renal disease and death. Clin J Am Soc Nephrol. 2011;6:2444–2451. doi: 10.2215/CJN.00580111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH, Steffes MW; American Diabetes Association. Nephropathy in diabetes. Diabetes Care. 2004;27 Suppl 1:S79–S83. doi: 10.2337/diacare.27.2007.s79. [DOI] [PubMed] [Google Scholar]
- 10.Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63:713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 11.Icks A, Koch M. Epidemiology of chronic kidney disease in diseases. In: Wolf G, editor. Diabetes and kidney disease. West Sussex, UK: John Wiley & Sons Ltd; 2013. pp. 14–28. [Google Scholar]
- 12.Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR; UKPDS Study Group. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes. 2006;55:1832–1839. doi: 10.2337/db05-1620. [DOI] [PubMed] [Google Scholar]
- 13.Parving HH, Lewis JB, Ravid M, Remuzzi G, Hunsicker LG; DEMAND investigators. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int. 2006;69:2057–2063. doi: 10.1038/sj.ki.5000377. [DOI] [PubMed] [Google Scholar]
- 14.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Ishani A, Johansen K, Kasiske BL, Kutner N, Liu J, et al. US Renal Data System 2013 Annual Data Report. Am J Kidney Dis. 2014;63:A7. doi: 10.1053/j.ajkd.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Y, Echouffo-Tcheugui JB, Gu JJ, Ruan XN, Zhao GM, Xu WH, Yang LM, Zhang H, Qiu H, Narayan KM, et al. Prevalence of chronic kidney disease across levels of glycemia among adults in Pudong New Area, Shanghai, China. BMC Nephrol. 2013;14:253. doi: 10.1186/1471-2369-14-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rathmann W, Haastert B, Icks A, Giani G, Holle R, Meisinger C, Mielck A; KORA Study Group. Sex differences in the associations of socioeconomic status with undiagnosed diabetes mellitus and impaired glucose tolerance in the elderly population: the KORA Survey 2000. Eur J Public Health. 2005;15:627–633. doi: 10.1093/eurpub/cki037. [DOI] [PubMed] [Google Scholar]
- 17.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 18.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 19.Imai E, Horio M, Yamagata K, Iseki K, Hara S, Ura N, Kiyohara Y, Makino H, Hishida A, Matsuo S. Slower decline of glomerular filtration rate in the Japanese general population: a longitudinal 10-year follow-up study. Hypertens Res. 2008;31:433–441. doi: 10.1291/hypres.31.433. [DOI] [PubMed] [Google Scholar]
- 20.Matsushita K, Selvin E, Bash LD, Franceschini N, Astor BC, Coresh J. Change in estimated GFR associates with coronary heart disease and mortality. J Am Soc Nephrol. 2009;20:2617–2624. doi: 10.1681/ASN.2009010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kronborg J, Solbu M, Njølstad I, Toft I, Eriksen BO, Jenssen T. Predictors of change in estimated GFR: a population-based 7-year follow-up from the Tromso study. Nephrol Dial Transplant. 2008;23:2818–2826. doi: 10.1093/ndt/gfn148. [DOI] [PubMed] [Google Scholar]
- 22.Hemmelgarn BR, Zhang J, Manns BJ, Tonelli M, Larsen E, Ghali WA, Southern DA, McLaughlin K, Mortis G, Culleton BF. Progression of kidney dysfunction in the community-dwelling elderly. Kidney Int. 2006;69:2155–2161. doi: 10.1038/sj.ki.5000270. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Gassman JJ, Hall PM, Walker WG. Assessing the progression of renal disease in clinical studies: effects of duration of follow-up and regression to the mean. Modification of Diet in Renal Disease (MDRD) Study Group. J Am Soc Nephrol. 1991;1:1087–1094. doi: 10.1681/ASN.V191087. [DOI] [PubMed] [Google Scholar]
- 24.Wright JT, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 25.Eriksen BO, Ingebretsen OC. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int. 2006;69:375–382. doi: 10.1038/sj.ki.5000058. [DOI] [PubMed] [Google Scholar]
- 26.Levin A, Djurdjev O, Beaulieu M, Er L. Variability and risk factors for kidney disease progression and death following attainment of stage 4 CKD in a referred cohort. Am J Kidney Dis. 2008;52:661–671. doi: 10.1053/j.ajkd.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 27.Al-Aly Z, Zeringue A, Fu J, Rauchman MI, McDonald JR, El-Achkar TM, Balasubramanian S, Nurutdinova D, Xian H, Stroupe K, et al. Rate of kidney function decline associates with mortality. J Am Soc Nephrol. 2010;21:1961–1969. doi: 10.1681/ASN.2009121210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shlipak MG, Katz R, Kestenbaum B, Siscovick D, Fried L, Newman A, Rifkin D, Sarnak MJ. Rapid decline of kidney function increases cardiovascular risk in the elderly. J Am Soc Nephrol. 2009;20:2625–2630. doi: 10.1681/ASN.2009050546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng TY, Wen SF, Astor BC, Tao XG, Samet JM, Wen CP. Mortality risks for all causes and cardiovascular diseases and reduced GFR in a middle-aged working population in Taiwan. Am J Kidney Dis. 2008;52:1051–1060. doi: 10.1053/j.ajkd.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 30.Rifkin DE, Shlipak MG, Katz R, Fried LF, Siscovick D, Chonchol M, Newman AB, Sarnak MJ. Rapid kidney function decline and mortality risk in older adults. Arch Intern Med. 2008;168:2212–2218. doi: 10.1001/archinte.168.20.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrassy KM. Comments on ‘KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease’. Kidney Int. 2013;84:622–623. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 32.Raymond NT, Paul O’Hare J, Bellary S, Kumar S, Jones A, Barnett AH; UKADS Study Group. Comparative risk of microalbuminuria and proteinuria in UK residents of south Asian and white European ethnic background with type 2 diabetes: a report from UKADS. Curr Med Res Opin. 2011;27 Suppl 3:47–55. doi: 10.1185/03007995.2011.614937. [DOI] [PubMed] [Google Scholar]
- 33.Dreyer G, Hull S, Mathur R, Chesser A, Yaqoob MM. Progression of chronic kidney disease in a multi-ethnic community cohort of patients with diabetes mellitus. Diabet Med. 2013;30:956–963. doi: 10.1111/dme.12197. [DOI] [PubMed] [Google Scholar]
- 34.Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Herzog C, Ishani A, Johansen K, Kasiske B, Kutner N, et al. US Renal Data System 2012 Annual Data Report. Am J Kidney Dis. 2013;61:A7, e1–476. doi: 10.1053/j.ajkd.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 35.Pezzolesi MG, Krolewski AS. The genetic risk of kidney disease in type 2 diabetes. Med Clin North Am. 2013;97:91–107. doi: 10.1016/j.mcna.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Böger CA, Mertens PR. Genetic risk factors for diabetic nephropathy. In: Wolf G, editor. Diabetes and kidney disease. West Sussex, UK: John Wiley & Sons Ltd; 2013. pp. 9–44. [Google Scholar]
- 37.Shimazaki A, Kawamura Y, Kanazawa A, Sekine A, Saito S, Tsunoda T, Koya D, Babazono T, Tanaka Y, Matsuda M, et al. Genetic variations in the gene encoding ELMO1 are associated with susceptibility to diabetic nephropathy. Diabetes. 2005;54:1171–1178. doi: 10.2337/diabetes.54.4.1171. [DOI] [PubMed] [Google Scholar]
- 38.Leak TS, Perlegas PS, Smith SG, Keene KL, Hicks PJ, Langefeld CD, Mychaleckyj JC, Rich SS, Kirk JK, Freedman BI, et al. Variants in intron 13 of the ELMO1 gene are associated with diabetic nephropathy in African Americans. Ann Hum Genet. 2009;73:152–159. doi: 10.1111/j.1469-1809.2008.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iyengar SK, Abboud HE, Goddard KA, Saad MF, Adler SG, Arar NH, Bowden DW, Duggirala R, Elston RC, Hanson RL, et al. Genome-wide scans for diabetic nephropathy and albuminuria in multiethnic populations: the family investigation of nephropathy and diabetes (FIND) Diabetes. 2007;56:1577–1585. doi: 10.2337/db06-1154. [DOI] [PubMed] [Google Scholar]
- 40.Vardarli I, Baier LJ, Hanson RL, Akkoyun I, Fischer C, Rohmeiss P, Basci A, Bartram CR, Van Der Woude FJ, Janssen B. Gene for susceptibility to diabetic nephropathy in type 2 diabetes maps to 18q22.3-23. Kidney Int. 2002;62:2176–2183. doi: 10.1046/j.1523-1755.2002.00663.x. [DOI] [PubMed] [Google Scholar]
- 41.McDonough CW, Hicks PJ, Lu L, Langefeld CD, Freedman BI, Bowden DW. The influence of carnosinase gene polymorphisms on diabetic nephropathy risk in African-Americans. Hum Genet. 2009;126:265–275. doi: 10.1007/s00439-009-0667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maeda S, Kobayashi MA, Araki S, Babazono T, Freedman BI, Bostrom MA, Cooke JN, Toyoda M, Umezono T, Tarnow L, et al. A single nucleotide polymorphism within the acetyl-coenzyme A carboxylase beta gene is associated with proteinuria in patients with type 2 diabetes. PLoS Genet. 2010;6:e1000842. doi: 10.1371/journal.pgen.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maeda S, Araki S, Babazono T, Toyoda M, Umezono T, Kawai K, Imanishi M, Uzu T, Watada H, Suzuki D, et al. Replication study for the association between four Loci identified by a genome-wide association study on European American subjects with type 1 diabetes and susceptibility to diabetic nephropathy in Japanese subjects with type 2 diabetes. Diabetes. 2010;59:2075–2079. doi: 10.2337/db10-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka N, Babazono T, Saito S, Sekine A, Tsunoda T, Haneda M, Tanaka Y, Fujioka T, Kaku K, Kawamori R, et al. Association of solute carrier family 12 (sodium/chloride) member 3 with diabetic nephropathy, identified by genome-wide analyses of single nucleotide polymorphisms. Diabetes. 2003;52:2848–2853. doi: 10.2337/diabetes.52.11.2848. [DOI] [PubMed] [Google Scholar]
- 45.Awadallah S, Hamad M. A study of haptoglobin phenotypes in patients with chronic renal failure. Ann Clin Biochem. 2003;40:680–683. doi: 10.1258/000456303770367298. [DOI] [PubMed] [Google Scholar]
- 46.Conway BR, Savage DA, Brady HR, Maxwell AP. Association between haptoglobin gene variants and diabetic nephropathy: haptoglobin polymorphism in nephropathy susceptibility. Nephron Exp Nephrol. 2007;105:e75–e79. doi: 10.1159/000098563. [DOI] [PubMed] [Google Scholar]
- 47.Nakhoul FM, Miller-Lotan R, Awaad H, Asleh R, Levy AP. Hypothesis--haptoglobin genotype and diabetic nephropathy. Nat Clin Pract Nephrol. 2007;3:339–344. doi: 10.1038/ncpneph0467. [DOI] [PubMed] [Google Scholar]
- 48.Asleh R, Levy AP. In vivo and in vitro studies establishing haptoglobin as a major susceptibility gene for diabetic vascular disease. Vasc Health Risk Manag. 2005;1:19–28. doi: 10.2147/vhrm.1.1.19.58930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jindal A, Garcia-Touza M, Jindal N, Whaley-Connell A, Sowers JR. Diabetic kidney disease and the cardiorenal syndrome: old disease, new perspectives. Endocrinol Metab Clin North Am. 2013;42:789–808. doi: 10.1016/j.ecl.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scheven L, Halbesma N, de Jong PE, de Zeeuw D, Bakker SJ, Gansevoort RT. Predictors of progression in albuminuria in the general population: results from the PREVEND cohort. PLoS One. 2013;8:e61119. doi: 10.1371/journal.pone.0061119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanaka Y, Daida H, Imai Y, Miyauchi K, Sato Y, Hiwatari M, Kitagawa A, Kishimoto J, Yamazaki T, Kawamori R. Morning home blood pressure may be a significant marker of nephropathy in Japanese patients with type 2 diabetes: ADVANCED-J study 1. Hypertens Res. 2009;32:770–774. doi: 10.1038/hr.2009.96. [DOI] [PubMed] [Google Scholar]
- 52.Bash LD, Selvin E, Steffes M, Coresh J, Astor BC. Poor glycemic control in diabetes and the risk of incident chronic kidney disease even in the absence of albuminuria and retinopathy: Atherosclerosis Risk in Communities (ARIC) Study. Arch Intern Med. 2008;168:2440–2447. doi: 10.1001/archinte.168.22.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin CC, Liu CS, Li CI, Lin WY, Lai MM, Lin T, Chang PC, Lee YD, Chen CC, Lin CH, et al. The relation of metabolic syndrome according to five definitions to cardiovascular risk factors--a population-based study. BMC Public Health. 2009;9:484. doi: 10.1186/1471-2458-9-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Al-Rubeaan K, Youssef AM, Subhani SN, Ahmad NA, Al-Sharqawi AH, Al-Mutlaq HM, David SK, AlNaqeb D. Diabetic nephropathy and its risk factors in a society with a type 2 diabetes epidemic: a Saudi National Diabetes Registry-based study. PLoS One. 2014;9:e88956. doi: 10.1371/journal.pone.0088956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Hauteclocque A, Ragot S, Slaoui Y, Gand E, Miot A, Sosner P, Halimi JM, Zaoui P, Rigalleau V, Roussel R, Saulnier PJ, Hadjadj Samy S; The SURDIAGENE Study group. The influence of sex on renal function decline in people with Type 2 diabetes. Diabet Med. 2014;31:1121–1128. doi: 10.1111/dme.12478. [DOI] [PubMed] [Google Scholar]
- 56.Ajayi S, Mamven M, Ojji D. eGFR and chronic kidney disease stages among newly diagnosed asymptomatic hypertensives and diabetics seen in a tertiary health center in Nigeria. Ethn Dis. 2014;24:220–225. [PubMed] [Google Scholar]
- 57.Macisaac RJ, Ekinci EI, Jerums G. Markers of and risk factors for the development and progression of diabetic kidney disease. Am J Kidney Dis. 2014;63:S39–S62. doi: 10.1053/j.ajkd.2013.10.048. [DOI] [PubMed] [Google Scholar]
- 58.Ahmed MA, Kishore G, Khader HA, Kasturirangan MN. Risk factors and management of diabetic nephropathy. Saudi J Kidney Dis Transpl. 2013;24:1242–1247. doi: 10.4103/1319-2442.121310. [DOI] [PubMed] [Google Scholar]
- 59.Ayodele OE, Alebiosu CO, Salako BL. Diabetic nephropathy--a review of the natural history, burden, risk factors and treatment. J Natl Med Assoc. 2004;96:1445–1454. [PMC free article] [PubMed] [Google Scholar]
- 60.van den Berg E, Hospers FA, Navis G, Engberink MF, Brink EJ, Geleijnse JM, van Baak MA, Gans RO, Bakker SJ. Dietary acid load and rapid progression to end-stage renal disease of diabetic nephropathy in Westernized South Asian people. J Nephrol. 2011;24:11–17. doi: 10.5301/jn.2010.5711. [DOI] [PubMed] [Google Scholar]
- 61.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 62.Ritz E. Metabolic syndrome and kidney disease. Blood Purif. 2008;26:59–62. doi: 10.1159/000110566. [DOI] [PubMed] [Google Scholar]
- 63.Chawla V, Greene T, Beck GJ, Kusek JW, Collins AJ, Sarnak MJ, Menon V. Hyperlipidemia and long-term outcomes in nondiabetic chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:1582–1587. doi: 10.2215/CJN.01450210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee PH, Chang HY, Tung CW, Hsu YC, Lei CC, Chang HH, Yang HF, Lu LC, Jong MC, Chen CY, et al. Hypertriglyceridemia: an independent risk factor of chronic kidney disease in Taiwanese adults. Am J Med Sci. 2009;338:185–189. doi: 10.1097/MAJ.0b013e3181a92804. [DOI] [PubMed] [Google Scholar]
- 65.Tien KJ, Tu ST, Chen HC, Hsiao JY, Hsieh MC. Triglycerides are independently associated with albuminuria in Taiwanese Type 2 diabetic patients. J Endocrinol Invest. 2012;35:800–803. doi: 10.3275/8060. [DOI] [PubMed] [Google Scholar]
- 66.Koch M, Beien A, Fusshã Ller A, Zitta S, Haastert B, Trapp R. Impact of age, body mass index, insulin resistance and proteinuria on the kidney function in obese patients with Type 2 diabetes and renal insufficiency. Clin Nephrol. 2008;69:10–17. doi: 10.5414/cnp69010. [DOI] [PubMed] [Google Scholar]
- 67.Wang YL, Shu KH, Yang MF, Yang WC, Wu MJ, Lin TM, Chen CH. The impact of body weight management in chronic kidney disease patients with obesity. J Ren Nutr. 2013;23:372–379. doi: 10.1053/j.jrn.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 68.Fu CP, Lee IT, Sheu WH, Lee WJ, Liang KW, Lee WL, Lin SY. The levels of circulating and urinary monocyte chemoattractant protein-1 are associated with chronic renal injury in obese men. Clin Chim Acta. 2012;413:1647–1651. doi: 10.1016/j.cca.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 69.Zhang J, Chen Y, Xu Y, Li M, Wang T, Xu B, Sun J, Xu M, Lu J, Bi Y. Low-Grade Albuminuria Is Associated with Metabolic Syndrome and Its Components in Middle-Aged and Elderly Chinese Population. PLoS One. 2013;8:e65597. doi: 10.1371/journal.pone.0065597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zoppini G, Targher G, Chonchol M, Ortalda V, Negri C, Stoico V, Bonora E. Predictors of estimated GFR decline in patients with type 2 diabetes and preserved kidney function. Clin J Am Soc Nephrol. 2012;7:401–408. doi: 10.2215/CJN.07650711. [DOI] [PubMed] [Google Scholar]
- 71.Nosadini R, Tonolo G. Blood glucose and lipid control as risk factors in the progression of renal damage in type 2 diabetes. J Nephrol. 2003;16 Suppl 7:S42–S47. [PubMed] [Google Scholar]
- 72.Sheen YJ, Lin JL, Li TC, Bau CT, Sheu WH. Peripheral arterial stiffness is independently associated with a rapid decline in estimated glomerular filtration rate in patients with type 2 diabetes. Biomed Res Int. 2013;2013:309294. doi: 10.1155/2013/309294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoefield RA, Kalra PA, Baker PG, Sousa I, Diggle PJ, Gibson MJ, O’Donoghue DJ, Middleton RJ, New JP. The use of eGFR and ACR to predict decline in renal function in people with diabetes. Nephrol Dial Transplant. 2011;26:887–892. doi: 10.1093/ndt/gfq526. [DOI] [PubMed] [Google Scholar]
- 74.Clark WF, Macnab JJ, Sontrop JM, Jain AK, Moist L, Salvadori M, Suri R, Garg AX. Dipstick proteinuria as a screening strategy to identify rapid renal decline. J Am Soc Nephrol. 2011;22:1729–1736. doi: 10.1681/ASN.2010111217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luo Y, Li X, Li J, Wang X, Xu Y, Qiao Y, Hu D, Ma Y. Peripheral arterial disease, chronic kidney disease, and mortality: the Chinese Ankle Brachial Index Cohort Study. Vasc Med. 2010;15:107–112. doi: 10.1177/1358863X09357230. [DOI] [PubMed] [Google Scholar]
- 76.Ruggenenti P, Perna A, Ganeva M, Ene-Iordache B, Remuzzi G; BENEDICT Study Group. Impact of blood pressure control and angiotensin-converting enzyme inhibitor therapy on new-onset microalbuminuria in type 2 diabetes: a post hoc analysis of the BENEDICT trial. J Am Soc Nephrol. 2006;17:3472–3481. doi: 10.1681/ASN.2006060560. [DOI] [PubMed] [Google Scholar]
- 77.Whelton PK, Perneger TV, He J, Klag MJ. The role of blood pressure as a risk factor for renal disease: a review of the epidemiologic evidence. J Hum Hypertens. 1996;10:683–689. [PubMed] [Google Scholar]
- 78.Haroun MK, Jaar BG, Hoffman SC, Comstock GW, Klag MJ, Coresh J. Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington County, Maryland. J Am Soc Nephrol. 2003;14:2934–2941. doi: 10.1097/01.asn.0000095249.99803.85. [DOI] [PubMed] [Google Scholar]
- 79.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 80.Bakris GL, Weir MR, Shanifar S, Zhang Z, Douglas J, van Dijk DJ, Brenner BM; RENAAL Study Group. Effects of blood pressure level on progression of diabetic nephropathy: results from the RENAAL study. Arch Intern Med. 2003;163:1555–1565. doi: 10.1001/archinte.163.13.1555. [DOI] [PubMed] [Google Scholar]
- 81.Okada H, Fukui M, Tanaka M, Matsumoto S, Mineoka Y, Nakanishi N, Asano M, Yamazaki M, Hasegawa G, Nakamura N. Visit-to-visit blood pressure variability is a novel risk factor for the development and progression of diabetic nephropathy in patients with type 2 diabetes. Diabetes Care. 2013;36:1908–1912. doi: 10.2337/dc12-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sheen YJ, Lin JL, Lee IT, Hsu YN, Li TC, Sheu WH. Low estimated glomerular filtration rate is a major determinant of low ankle-brachial index and toe-brachial index in type 2 diabetes. Angiology. 2012;63:55–61. doi: 10.1177/0003319711406709. [DOI] [PubMed] [Google Scholar]
- 83.Yamashita T, Makino H, Nakatani R, Ohata Y, Miyamoto Y, Kishimoto I. Renal insufficiency without albuminuria is associated with peripheral artery atherosclerosis and lipid metabolism disorders in patients with type 2 diabetes. J Atheroscler Thromb. 2013;20:790–797. doi: 10.5551/jat.15669. [DOI] [PubMed] [Google Scholar]
- 84.Bouchi R, Babazono T, Mugishima M, Yoshida N, Nyumura I, Toya K, Hanai K, Tanaka N, Ishii A, Uchigata Y, et al. Arterial stiffness is associated with incident albuminuria and decreased glomerular filtration rate in type 2 diabetic patients. Diabetes Care. 2011;34:2570–2575. doi: 10.2337/dc11-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen SC, Lin TH, Hsu PC, Chang JM, Lee CS, Tsai WC, Su HM, Voon WC, Chen HC. Impaired left ventricular systolic function and increased brachial-ankle pulse-wave velocity are independently associated with rapid renal function progression. Hypertens Res. 2011;34:1052–1058. doi: 10.1038/hr.2011.95. [DOI] [PubMed] [Google Scholar]
- 86.Li DM, Zhang Y, Ding B, Liu BL, Jiang LL, Xing CY, Ma JH. [The association between vitamin D deficiency and diabetic nephropathy in type 2 diabetic patients] Zhonghua Neike Zazhi. 2013;52:970–974. [PubMed] [Google Scholar]
- 87.de Boer IH, Katz R, Chonchol M, Ix JH, Sarnak MJ, Shlipak MG, Siscovick DS, Kestenbaum B. Serum 25-hydroxyvitamin D and change in estimated glomerular filtration rate. Clin J Am Soc Nephrol. 2011;6:2141–2149. doi: 10.2215/CJN.02640311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Trevisan R, Vedovato M, Mazzon C, Coracina A, Iori E, Tiengo A, Del Prato S. Concomitance of diabetic retinopathy and proteinuria accelerates the rate of decline of kidney function in type 2 diabetic patients. Diabetes Care. 2002;25:2026–2031. doi: 10.2337/diacare.25.11.2026. [DOI] [PubMed] [Google Scholar]
- 89.Sakata M, Oniki K, Kita A, Kajiwara A, Uchiyashiki Y, Saruwatari J, Yoshida A, Jinnouchi H, Nakagawa K. Clinical features associated with a rapid decline in renal function among Japanese patients with type 2 diabetes mellitus: microscopic hematuria coexisting with diabetic retinopathy. Diabetes Res Clin Pract. 2013;100:e39–e41. doi: 10.1016/j.diabres.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 90.Tahrani AA, Dubb K, Raymond NT, Begum S, Altaf QA, Sadiqi H, Piya MK, Stevens MJ. Cardiac autonomic neuropathy predicts renal function decline in patients with type 2 diabetes: a cohort study. Diabetologia. 2014;57:1249–1256. doi: 10.1007/s00125-014-3211-2. [DOI] [PubMed] [Google Scholar]
- 91.Ruggenenti P, Porrini EL, Gaspari F, Motterlini N, Cannata A, Carrara F, Cella C, Ferrari S, Stucchi N, Parvanova A, et al. Glomerular hyperfiltration and renal disease progression in type 2 diabetes. Diabetes Care. 2012;35:2061–2068. doi: 10.2337/dc11-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Perkins BA, Nelson RG, Ostrander BE, Blouch KL, Krolewski AS, Myers BD, Warram JH. Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: results of a 4-year follow-up study. J Am Soc Nephrol. 2005;16:1404–1412. doi: 10.1681/ASN.2004100854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Macisaac RJ, Tsalamandris C, Thomas MC, Premaratne E, Panagiotopoulos S, Smith TJ, Poon A, Jenkins MA, Ratnaike SI, Power DA, et al. The accuracy of cystatin C and commonly used creatinine-based methods for detecting moderate and mild chronic kidney disease in diabetes. Diabet Med. 2007;24:443–448. doi: 10.1111/j.1464-5491.2007.02112.x. [DOI] [PubMed] [Google Scholar]
- 94.Kramer HJ, Nguyen QD, Curhan G, Hsu CY. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA. 2003;289:3273–3277. doi: 10.1001/jama.289.24.3273. [DOI] [PubMed] [Google Scholar]
- 95.Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, Patel A, Cass A, Neal B, Poulter N, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20:1813–1821. doi: 10.1681/ASN.2008121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reeves WB, Rawal BB, Abdel-Rahman EM, Awad AS. Therapeutic Modalities in Diabetic Nephropathy: Future Approaches. Open J Nephrol. 2012;2:5–18. doi: 10.4236/ojneph.2012.22002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ruggenenti P, Cravedi P, Remuzzi G. The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nat Rev Nephrol. 2010;6:319–330. doi: 10.1038/nrneph.2010.58. [DOI] [PubMed] [Google Scholar]
- 98.Taal MW, Brenner BM. Renoprotective benefits of RAS inhibition: from ACEI to angiotensin II antagonists. Kidney Int. 2000;57:1803–1817. doi: 10.1046/j.1523-1755.2000.00031.x. [DOI] [PubMed] [Google Scholar]
- 99.Chen SC, Tseng CH. Dyslipidemia, kidney disease, and cardiovascular disease in diabetic patients. Rev Diabet Stud. 2013;10:88–100. doi: 10.1900/RDS.2013.10.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Holtkamp FA, de Zeeuw D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJ, Parving HH, Brenner BM, Shahinfar S, Lambers Heerspink HJ. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 2011;80:282–287. doi: 10.1038/ki.2011.79. [DOI] [PubMed] [Google Scholar]
- 101.American Diabetes Association. Standards of medical care in diabetes--2013. Diabetes Care. 2013;36 Suppl 1:S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mann JF, Anderson C, Gao P, Gerstein HC, Boehm M, Rydén L, Sleight P, Teo KK, Yusuf S; ONTARGET investigators. Dual inhibition of the renin-angiotensin system in high-risk diabetes and risk for stroke and other outcomes: results of the ONTARGET trial. J Hypertens. 2013;31:414–421. doi: 10.1097/HJH.0b013e32835bf7b0. [DOI] [PubMed] [Google Scholar]
- 103.Gaede P, Tarnow L, Vedel P, Parving HH, Pedersen O. Remission to normoalbuminuria during multifactorial treatment preserves kidney function in patients with type 2 diabetes and microalbuminuria. Nephrol Dial Transplant. 2004;19:2784–2788. doi: 10.1093/ndt/gfh470. [DOI] [PubMed] [Google Scholar]
- 104.Gaede P, Vedel P, Parving HH, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet. 1999;353:617–622. doi: 10.1016/S0140-6736(98)07368-1. [DOI] [PubMed] [Google Scholar]
- 105.Gaede PH. Intensified multifactorial intervention in patients with type 2 diabetes and microalbuminuria: rationale and effect on late-diabetic complications. Dan Med Bull. 2006;53:258–284. [PubMed] [Google Scholar]
- 106.Tu ST, Chang SJ, Chen JF, Tien KJ, Hsiao JY, Chen HC, Hsieh MC. Prevention of diabetic nephropathy by tight target control in an asian population with type 2 diabetes mellitus: a 4-year prospective analysis. Arch Intern Med. 2010;170:155–161. doi: 10.1001/archinternmed.2009.471. [DOI] [PubMed] [Google Scholar]
- 107.Chan JC, So WY, Yeung CY, Ko GT, Lau IT, Tsang MW, Lau KP, Siu SC, Li JK, Yeung VT, Leung WY, Tong PC; SURE Study Group. Effects of structured versus usual care on renal endpoint in type 2 diabetes: the SURE study: a randomized multicenter translational study. Diabetes Care. 2009;32:977–982. doi: 10.2337/dc08-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bos M, Agyemang C. Prevalence and complications of diabetes mellitus in Northern Africa, a systematic review. BMC Public Health. 2013;13:387. doi: 10.1186/1471-2458-13-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Herman WH, Aubert RE, Engelgau MM, Thompson TJ, Ali MA, Sous ES, Hegazy M, Badran A, Kenny SJ, Gunter EW, et al. Diabetes mellitus in Egypt: glycaemic control and microvascular and neuropathic complications. Diabet Med. 1998;15:1045–1051. doi: 10.1002/(SICI)1096-9136(1998120)15:12<1045::AID-DIA696>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 110.Elbagir MN, Eltom MA, Mahadi EO, Berne C. Pattern of long-term complications in Sudanese insulin-treated diabetic patients. Diabetes Res Clin Pract. 1995;30:59–67. doi: 10.1016/0168-8227(95)01146-3. [DOI] [PubMed] [Google Scholar]
- 111.Hamed SA, Amine NF, Galal GM, Helal SR, Tag El-Din LM, Shawky OA, Ahmed EA, Abdel Rahman MS. Vascular risks and complications in diabetes mellitus: the role of helicobacter pylori infection. J Stroke Cerebrovasc Dis. 2008;17:86–94. doi: 10.1016/j.jstrokecerebrovasdis.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 112.Chadban SJ, Briganti EM, Kerr PG, Dunstan DW, Welborn TA, Zimmet PZ, Atkins RC. Prevalence of kidney damage in Australian adults: The AusDiab kidney study. J Am Soc Nephrol. 2003;14:S131–S138. doi: 10.1097/01.asn.0000070152.11927.4a. [DOI] [PubMed] [Google Scholar]
- 113.Unnikrishnan RI, Rema M, Pradeepa R, Deepa M, Shanthirani CS, Deepa R, Mohan V. Prevalence and risk factors of diabetic nephropathy in an urban South Indian population: the Chennai Urban Rural Epidemiology Study (CURES 45) Diabetes Care. 2007;30:2019–2024. doi: 10.2337/dc06-2554. [DOI] [PubMed] [Google Scholar]
- 114.Lin CH, Yang WC, Tsai ST, Tung TH, Chou P. A community-based study of chronic kidney disease among type 2 diabetics in Kinmen, Taiwan. Diabetes Res Clin Pract. 2007;75:306–312. doi: 10.1016/j.diabres.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 115.Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, Shan Z, Liu J, Tian H, Ji Q, Zhu D, Ge J, Lin L, Chen L, Guo X, Zhao Z, Li Q, Zhou Z, Shan G, He J; China National Diabetes and Metabolic Disorders Study Group. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–1101. [Google Scholar]
- 116.Lou Arnal LM, Campos Gutiérrez B, Cuberes Izquierdo M, Gracia García O, Turón Alcaine JM, Bielsa García S, Gimeno Orna JA, Boned Juliani B, Sanjuán Hernández-French A. [Prevalence of chronic kidney disease in patients with type 2 diabetes mellitus treated in primary care] Nefrologia. 2010;30:552–556. doi: 10.3265/Nefrologia.pre2010.Jun.10260. [DOI] [PubMed] [Google Scholar]
- 117.Detournay B, Simon D, Guillausseau PJ, Joly D, Verges B, Attali C, Clement O, Briand Y, Delaitre O. Chronic kidney disease in type 2 diabetes patients in France: prevalence, influence of glycaemic control and implications for the pharmacological management of diabetes. Diabetes Metab. 2012;38:102–112. doi: 10.1016/j.diabet.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 118.Tsai YC, Tsai JC, Chiu YW, Kuo HT, Chen SC, Hwang SJ, Chen TH, Kuo MC, Chen HC. Is fluid overload more important than diabetes in renal progression in late chronic kidney disease? PLoS One. 2013;8:e82566. doi: 10.1371/journal.pone.0082566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Clark WF, Sontrop JM, Macnab JJ, Suri RS, Moist L, Salvadori M, Garg AX. Urine volume and change in estimated GFR in a community-based cohort study. Clin J Am Soc Nephrol. 2011;6:2634–2641. doi: 10.2215/CJN.01990211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pham PC, Pham PM, Pham PA, Pham SV, Pham HV, Miller JM, Yanagawa N, Pham PT. Lower serum magnesium levels are associated with more rapid decline of renal function in patients with diabetes mellitus type 2. Clin Nephrol. 2005;63:429–436. doi: 10.5414/cnp63429. [DOI] [PubMed] [Google Scholar]


