Abstract
Background
Apical Membrane antigen 1 (AMA-1) is positioned on the surface of merozoite and it may play a role in attack to red blood cells. The main aim of present study was to determine the genetic variation, as well as, to detect of selection at domain I of AMA-1 gene Plasmodium vivax isolates in Iran.
Methods
Blood samples were collected from 58 patients positive for P. vivax, mono infection and the domain I of AMA-1 gene was amplified by nested PCR and then sequenced.
Results
A total 33 different haplotypes were identified among 58 Iranian sequences. The 23 new haplotypes were determined in this study that was not reported previously in other regions of the world. There were totally observed 36 point mutations at the nucleotide level in the analyzed sequences. Sequences analyses indicated 25 amino acid changes at 20 positions in which 5 sites demonstrated thrimorphic polymorphism and the others were dimorphic in the domain I of the Iranian PvAMA-1 isolates.
Conclusion
Our findings indicated relatively high level of allelic diversity at the domain I of PvAMA-1 among P.vivax isolates of Iran. Since, PvAMA-1 is considering as vaccine candidate antigen, these data provide valuable information for the development of a PvAMA-1 based malaria vaccine.
Keywords: Plasmodium vivax, Malaria, Apical Membrane Antigen-1, Iran, Genetic variation
Introduction
Malaria remains one of the major infectious diseases in the world. Plasmodium vivax is the main cause of malaria in Asia, South America and Oceania and is also responsible for approximately 80-300 million cases of malaria per year in the world (1, 2). In 2011 totally 3271 clinical malaria cases were reported by Iranian Malaria Control Department in Disease Management Center and Prevention and more than 90% of them were related to P. vivax (Center for Diseases Management and Control, Tehran, Iran, unpublished data). Most of vivax malaria cases were reported from Sistan –Baluchistan and Hormozgan provinces located in southeast and south of Iran bordering with Pakistan and Afghanistan countries (3).
Because of widespread existence of chloroquine resistant parasites and resistant vectors against insecticides, beyond the lack of universal effective vaccine, so production of an efficient local, regional and universal vivax malaria vaccine is an important priority for control of the infection.
Apical Membrane Antigen 1 (AMA-1) is positioned on the surface of merozoite and may play a role in attack to red blood cells (4, 5). The AMA-1 protein has three domains and domain I plays an important role in stimulating immune system mechanisms against malaria infection.
AMA-1 is applied in combination with vaccines against both falciparum and vivax malaria in the world (6, 7). However, a significant pro-inflammatory immunity and antibodies were elicited by PvAMA-1 (8, 9). One of the challenging obstacles to access efficient vaccine against malaria is the genetic variation of vaccine candidate antigens including AMA-1 in various parts of the world. Detecting the polymorphisms of AMA-1 is the main priority to overcome this problem in the endemic malaria countries (10, 11).
Previous study on the AMA-1 Plasmodium falciparum in Iran revealed polymorphism and presence significantly non synonymous substitutions in Iranian isolates (12). The main objective of the present study was to determine the genetic variation, as well as, to detect of natural selection at domainI of AMA-1 gene P. vivax isolates in Iran. These data could be useful for manufacturing of vivax malaria vaccine.
Materials and Methods
Study area and blood sample collection
Iran with low malaria endemicity is located in the Middle- East Region. The malaria transmission mainly occurs in Sistan-Baluchistan and Hormozgan provinces located in the south and southeast of the country, respectively. Both autochthonous and imported malaria have been reported in these areas (13). This study was conducted in Sistan-Baluchistan and Hormozgan provinces. The majority of malaria cases (90%) caused by P. vivax occur in this regions (12).
Blood samples were collected from 58 confirmed patients positive for P. vivax, mono infection, by light microscopic examination of Giemsa-stained films at the health care centers in the study regions. After obtaining informed consent, 1.5 ml venous blood was taken from each patient in EDTA containing tubes and then stored in laboratory at -20 °C until DNA extraction. This study was approved by Ethical Committee of Alborz University of Medical Sciences, Karaj, Iran.
DNA extraction, PvAMA-1 amplification by PCR and DNA sequence
Plasmodium genomic DNA was obtained from 200 μl of the peripheral blood sample using a QIAamp DNA blood mini kit (Qiagen, Germany). A nested PCR amplification was carried out to amplify the partial PvAMA-1gene using Pfu DNA polymerase (Bioneer, Korea) and PVAF11 (5′-AGAATTCCAGC-TGGAAGATG-3′) and PVAR11 (5′- TCCTAAA-TTTTTACGGGGGCA-3′) primers (14). The amplification fragment for initial PCR was performed as follows: initial denaturation at 95 °C for 5 min followed by 40 cycles with denaturation at 94 °C for 1 min, annealing at 55 °C for 1 min, extension at 72 °C for 1 min and longation extension at 72 °C for 5 min. The first PCR product was applied as template in nested PCR to amplify the 390 bp region which contained domain I of the PfAMA-1 gene with PVAF5 (5′- GTTAGCTTCTTAAGACCTGTGGCT-3′) and PVAR5 (5′- TCCTAAAT-TTTTACGGGGGCA -3′) primers. The product was analyzed on 1.2% agarose gel. After purification, the gene fragments were directly sequenced on both strands by An-F and An-R primers in the Sequetech DNA Sequencing Service, California, USA.
Sequence analysis
Nucleotide sequences were checked using Chromas software version 2.33 (http://www.-technelysium.com.au/chromas.html) and aligned by ClustalW program (http://www.-genome.jp tools/clustalw).The nucleotide similarities was determined using Blast (http://-www.ncbi.nlm.nih.gov/blast) among the 58 Iranian sequences with previously deposited P. vivax AMA-1 in the GenBank database. The number of segregating (polymorphic) sites (S) and nucleotide diversity, Pi (π), which is the average number of nucleotide changes per site between any two sequences, as well as the number of haplotypes (H) and haplotype diversity (Hd) were obtained using DNASP software version 5.10.01 (15). The distribution of genetic variation across domain I of AMA-1 gene was measured by the sliding window method, estimating π on a moving window of 100 base pairs with a step size of 25 sites. Nucleotide variation and statistical analysis were investigated by MEGA software version 4.0 (16). The number of synonymous (dS) and nonsynonymous (dN) nucleotide diversity within species were estimated with use of the Nei and Gojobori method (17). The complete sequence of AMA-1 gene of P. vivax Sal1 strain (Accession No. XM_001615397) was applied to detect nucleotide and amino acid position numbers. Since, the Plasmodium cynmology is the closest species to P. vivax, so the partial AMA-1 sequence of this parasite (Accession No.X86099) was used to analyze some of neutrality tests as an outgroup species. Tajima’s D test statistics (18) was used to determine any departure from neutrality. To calculate the ratio of nonsynonymous to synonymous changes within and between species, the McDonald-Kreitman (MK) test was applied as a test of neutrality (19). Fisher’s exact test was used to detect any significant non randomness, and the deviation from randomness was calculated as the neutrality Index (20).
Results
A total of 58 allele sequences of the AMA- 1 gene were identified from all clinical vivax malaria patients. Out of 58 subjects 40 (69%) and 18 (31%) were belonged to Sistan-Baluchistan and Hormozgan provinces, respectively. The 389 bp in length, belonging to domain I (corresponding to 352-738 bp region of AMA-1 gene of Sal1 accession number: 156098862) was amplified by nested PCR and sequenced among the Iranian southern P. vivax isolates. There was no observed size polymorphism in nested PCR and all isolates exhibited approximately 400 bp fragments in gel electrophoresis. A total 33 different haplotypes were identified among 58 sequences (Fig. 1). Twenty three new haplotypes were determined in this study that had not been reported previously in other regions of the world. Haplotypes 6 and 28 illustrated the most frequency with 6 copies, whereas the other 21 haplotypes were unique with single copies (Fig. 1).We totally observed 36 point mutations at the nucleotide level in the analyzed sequences. Among 389 bp analyzed samples the 24 variable (polymorphic) nucleotide sites were exhibited with 6 and 18 sites singleton and parsimony informative, respectively. Among 24 variable nucleotides positions, only two sites were thrimorphic, while, the others were dimorphic. Haplotype and nucleotide diversity of the sequenced AMA-1 region are illustrated in Fig. 1. Sequences analyses indicated 25 amino acid changes at 20 positions in which 5 sites demonstrated thrimorphic polymorphism and the others were dimorphic in the domainI (Fig. 1). Our nucleotide sequences are available in the GenBank database, under the accession numbers JX 845357 to JX845414 (www.ncbi.nlm.nih.gov). The phylogenetic analysis of the 33 haplotypes reported in this research and selected isolates from several countries is indicated in Fig. 2.
Fig.1.

Polymorphic nucleotids (left) and amino acids (right) are exhibited in 58 Iranian PvAMA-1 domain I isolates. The total number of sequences for each haplotype is indicated in parenthesis. Nucleotide and amino acid position numbers are shown vertically above each variable site. Dots illustrate the same nucleotide and amino acid as in haplotype 1.H shows haplotype
Fig.2.
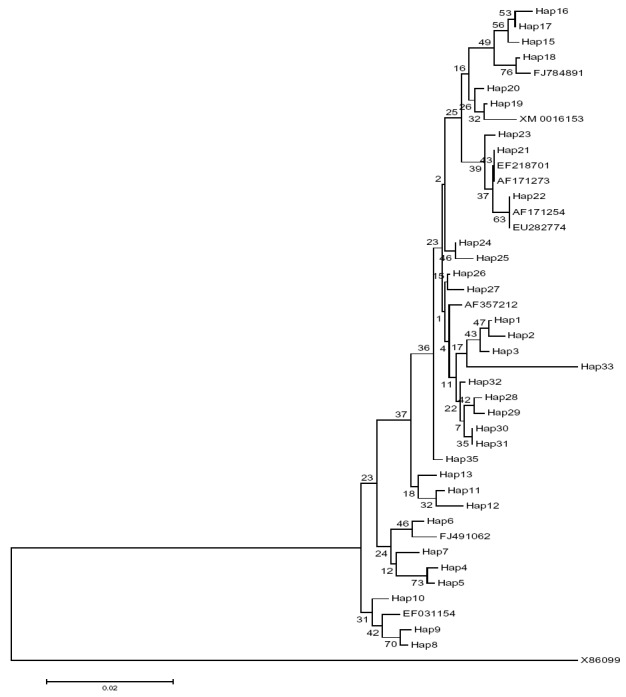
Neighbor-Joining tree of the AMA-1 haplotypes with use of Tamura 3-parameter distance in MEGA version 4.0 software. The numbers on the nods of tree illustrate the proportion of bootstrap values based on to 1000 replications. In addition, the haplotypes identified in this research (H1–H33) and a Strain of P. vivax Sal1 (XM_001615397), Eight isolates from several countries were used in phylogenetic tree construction, containing FJ784891: Thailand, aff171273: Philippine, EF218701: Seri Lankan, EU282774- FJ491062-AF171254: India, aff357212: Korea, EF031154: Brazil. The partial AMA-1 sequence of P.cynmology (X86099) was applied as an outgroup species
Nucleotide diversity and genetic differentiation
The haplotype and nucleotide diversity of 58 isolates were 0.969±0.01 and 0.01415±0.001, respectively. The average number of pairwise nucleotide differences (K) was 5.446. Further analyses with sliding window plot (window length 100 bp and a step size 25 bp) of DNASP revealed π diversity ranging from 0 to 0.02. Maximum diversity was appeared between nucleotide position 200 to 275 and then 300 to 350 bp (Fig 3). The rate of non-synonymous to synonymous substitution was 3.28 (Z-test: P < 0.05). The value of Tajimas D neutrality tests was -0.09741 by considering all mutation. Minimum rate of recombination events between adjacent polymorphic sites (RM) was 7, whereas R between adjacent sites (Ra) and per gene (Rb) was 0.08 and 5, respectively. The LD index R2 for Iranian isolates was also indicated to decline with increasing distance between pairs of nucleotides sites across the entire 389 bp region (Fig. 4).
Fig.3.
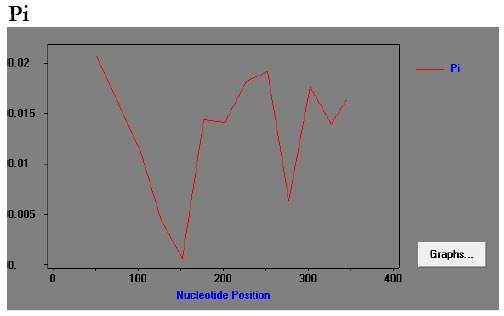
Sliding window plot of nucleotide variation. Pi(p) across the PvAMA-1 domain I in Iranian isolates with a window length 100 base pair and a step size of 25 base pair
Fig.4.
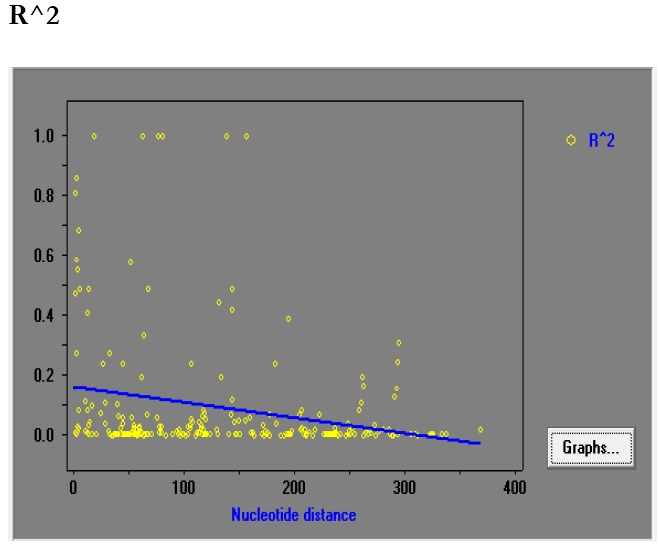
The association analysis of linkage disequilibrium (LD) index (R2) and nucleotide distance among the pairs of sites in 58 Iranian P.vivax isolates. The R2 values are plotted against the nucleotide distance with two-tailed Fisher’s exact test. The declines of LD index value with increasing nucleotide distances show that intragenic recombination may appear within the isolates. The pairs of sites that indicate statistically significant linkage disequilibrium are demonstrated as dark marks, while the other pairs of sites are characterized by faint-colored marks
Compare of nonsynonymous to synonymous substitution within species (Pn=25, Ps=1) and between species (Dn=13, Ds=31) MK test using indicated an access of intraspecific nonsynonymous changes relative to nonsynonymous substitution from the P. cynmology as outgroup species (neutrality index = 47.892 and P=0.000001 two-tailed fisher exact test).
Discussion
Analysis of genetic diversity supplies information about that how different haplotypes are generated and maintained in the population.
Since, immune selection pressure might play an important role in structuring the variation of highly polymorphic proteins, therefore genetic study of P. vivax population in endemic regions are necessary for development of effective vaccines. In this research, data were collected from Iranian isolates to contribute to make a better image of the vaccine candidate haplotypes circulating in Iran and to compare these results with previous studies.
Sequence analysis of the 58 Iranian sequences indicated that they were identified into 33 different haplotypes. The number of haplotypes identified among Iranian isolates is lower than those reported from Sri Lanken isolates (15 haplotypes in 23 isolates) (11) and Indian isolates (49 haplotypes in 61 isolates) (21) but is higher than Myanmar isolates (34 haplotypes in 76 isolates) (14) and Venezuela isolates (20 haplotype in 73 isolates) (22). Moreover, the genetic diversity, as calculated by the haplotype diversity at domain I AMA-1 locus, is higher among the Iranian P. vivax isolates (0.969) than Iranian P. falciparum isolates (0.958) (12). The more Haplotype diversity findings in P. vivax comparing with P. falciparum are consistent with the study conducted in Venezuela (22). Besides, analyses of the PvAMA-1 sequences illustrated that the level of π (nucleotide diversity) at domain I among the Iranian P. vivax isolates (π =0.01415) is higher than Sri Lanka (π =0.0092), while slightly lower than Myanmar (π=0.018) and India isolates (π=0.018) (11, 14, 21). Meanwhile it is nearly similar to reported polymorphism (π= 0.01406) in Sistan- Baluchistan Province of Iran isolates (23). This information could be indicative of genetic polymorphisms occurring at domain I in P. vivax isolates of southern Iran. The rate of non synonymous to synonymous substitution in the Iranian isolates was 3.287 for all 58 sequences identifying that the domain I of PvAMA-1 isolates is impressed by positive natural selection. It has been assumed that synonymous substitutions in malaria parasites are effectively neutral (24). Likewise, higher rate of higher nonsynonymous substitutions at domain I PvAMA-1 antigen could be considered as reaction of parasite to the host’s immune system and results from positive immune selection pressure for antigenic diversity (25).
The Tajimas D test (-0.09741) indicated departure from neutrality. A negative Tajimas D presently found, implies an access of low frequency polymorphism suggesting population size expansion and/or positive selection (18).
Indeed, our findings also demonstrated that recombination events have contributed to the observed diversity of PvAMA-1 among Iranian isolates collected from hypoendemic region. This is supported by decline of LD index R2 with increasing nucleotide distance. However, this finding suggests that intragenic recombination may play a role in the increased diversity observed at domain I of Iranian P. vivax isolates.
Findings of The MK test demonstrated significant departure from neutrality with an excess of intraspecific non synonymous changes relative to non synonymous polymorphisms from P. cynomolgi that indicate polymorphisms in the AMA-1gene are maintained by diversifying selection. Indeed, the occurred excess of non synonymous relative to synonymous substitutions is accounted as evidence of positive natural selection (26).
In our study area, the malaria transmission is unstable. Since, sexual development of parasite life cycle occurs in the mosquito midgut; it seems that sexual outcross and recombination events are related to the intensity of malaria transition.
However, these data may reveal that recombination plays a role in generating haplotype diversity in P.vivax AMA-1 in areas with low transmission rates. It seems that rare recombinant haplotypes emerged within the Iranian population is highly selectively advantageous or that new haplotypes emerged by recombination in areas of higher endemicity (including Pakistan and Afghanistan) moved into the Iranian population.
Similar findings are reported about P. falciparum AMA-1 from Thai and Iranian isolates (12, 27, 28). The sequences analysis indicates significant departure from neutrality on the basis of excess intra specific non synonymous substitution relative to non-synonymous changes from P. cynomology. These findings may suggest that polymorphisms found in domain I of PvAMA-1 are maintained by positive selection, mostly due to host immune pressure. The effects of intragenic recombination and natural selection on the observed genetic diversity in Iranian isolates are consistent with findings of previous studies (10, 11, 21, 22).
Considering the important role of natural selection in polymorphism of proteins, it cannot be accepted that a single strain could be applied as represent of haplotypes circulating worldwide and, therefore, as an outcome it will not be effective in all endemic regions.
The phylogenetic analysis of Iranian haplotypes with the numbers of previously haplotypes documented in GenBank (www. ncbi.nlm.nih.gov) indicates no significant geographic clustering of the isolates of Iran. It seems domainI PvAMA-1 exhibits limited genetic diversity within geographic regions. This finding is consistent with data reported in Seri Lanka (11). Since, the promising AMA-1 protein candidate for development vaccine is polymorphic and the generated immune reactions might be more effective against parasites into accounted in the particular vaccine. Therefore, our data together with previous studies on the PvAMA-1 locus based on the regional identification of AMA-1 sequences could be equally effective in protecting against vivax malaria in different endemic settings around the world.
Conclusion
We report here the genetic diversity of PvAMA-1 among Iranian isolates. Our findings indicate relatively high level of nucleotides and haplotype diversity at the domain I of PvAMA-1 among P.vivax isolates of Iran. Since, PvAMA-1 is considering as vaccine candidate antigen, these data are valuable for the development of a PvAMA-1 based malaria vaccine.
Acknowledgments
This study was financially supported by the Alborz University of Medical Sciences (Abzums), Karaj, Iran. The authors are also grateful to Mr Sakeni ,Mr Gorgige (Sistan-Baluchistan Provice) and Dr Shojaee and Mrs Salimi from the Department of Medical Parasitology and Mycology, School of Public Health and Institute of Public Health Research, Tehran University of Medical Sciences (TUMS). We would like to thank those individuals from the malaria endemic region of Iran, who kindly contributed to this study.
Footnotes
The authors declare that there is no conflict of interest.
References
- 1.Chenet SM, Schneider KA, Villegas L, Escalante AA. Local population structure of Plasmodium: impact on malaria control and elimination. Malar J. 2012;11:412. doi: 10.1186/1475-2875-11-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atemnkeng VA, Pink M, Schmitz-Spanke S, Wu XJ, Dong LL, Zhao KH, May C, Laufer S, Langer B, Kaiser A. Deoxyhypusine Hydroxylase from Plasmodium vivax, the Neglected Human Malaria Parasite: Molecular Cloning, Expression and Specific Inhibition by the 5-LOX Inhibitor Zileuton. PLoS One. 2013;8:e58318. doi: 10.1371/journal.pone.0058318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raeisi A, Gouya MM, Nadim A, Ranjbar M, Hasanzehi A, Fallahnezhad M, Sakeni M, Safari R, Saffari M, Mashyekhi M, Ahmadi Kahnali A, Mirkhani V, Almasian E, Faraji L, Paktinat Jalali B, Nikpour F. Determination of Malaria Epidemiological Status in Iran’s Malarious Areas as Baseline Information for Implementation of Malaria Elimination Program in Iran. Iranian J Publ Healt. 2013;42:326–33. [PMC free article] [PubMed] [Google Scholar]
- 4.Grynberg P, Fontes CJF, Hughes AL, Braga EM. Polymorphism at the apical membrane antigen-1 locus reflects the world population history of Plasmodium vivax. BMC Evol Biol. 2008;8:123. doi: 10.1186/1471-2148-8-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Triglia T, Healer J, Caruana SR, Hodder AN, Anders RF, Crabb BS, et al. Apical membrane antigen 1 plays a central role in erythrocyte invasion by Plasmodium species . Mol. Microbiol. 2000;38:706–18. doi: 10.1046/j.1365-2958.2000.02175.x. [DOI] [PubMed] [Google Scholar]
- 6.Bouillet LÉ, Dias MO, Dorigo NA, Moura AD, Russell B, et al. Long-Term Humoral and Cellular Immune Responses Elicited by a Heterologous Plasmodium vivax Apical Membrane Antigen 1 Protein Prime/Adenovirus Boost Immunization Protocol. Infect Immun. 2011;79:3642–52. doi: 10.1128/IAI.05048-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sagara I, Dicko A, Ellis RD, Fay MP, Diawara SI, Assadou MH, et al. A randomized controlled phase 2 trial of the blood stage AMA1-C1/alhydrogel malaria vaccine in children in Mali. Vaccine. 2009;27:3090–3098. doi: 10.1016/j.vaccine.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bueno LL, Morais CG, Soares IDS, Bouillet LEM, Bruna-Romero O, Fontes CJ, et al. Plasmodium vivax recombinant vaccine candidate AMA-1 plays an important role in adaptive immune response eliciting differentiation of dendritic cells. Vaccine. 2009;27:5581–88. doi: 10.1016/j.vaccine.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 9.Múfalo BC, Gentil F, Bargieri DY, Costa FT, Rodrigues MM, Soares IS. Plasmodium vivax apical membrane antigen-1: comparative recognition of different domains by antibodies induced during natural human infection. Microbes Infect. 2008;10:1266–73. doi: 10.1016/j.micinf.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 10.Putaporntip C, Jongwutiwes S, Grynberg P, Cui L, Hughes AL. Nucleotide sequence polymorphism at the apical membrane antigen-1 locus reveals population history of Plasmodium vivax in Thailand. Infect Genet Evol. 2009;9:1295–300. doi: 10.1016/j.meegid.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunasekera AM, Wickramarachchi T, Neafsey DE, Ganguli I, Perera L, Premaratne PH, et al. Genetic diversity and selection at the Plasmodium vivax Apical Membrane Antigen-1 (PvAMA-1) locus in a Sri Lankan population. Mol Biol Evol. 2007;24:939–47. doi: 10.1093/molbev/msm013. [DOI] [PubMed] [Google Scholar]
- 12.Mardani A, Keshavarz H, Heidari A, Hajjaran H, Raeisi A. Khorramizadeh MR.Genetic diversity and natural selection at the domain I of apical membrane antigen-1 (AMA-1) of Plasmodium falciparum in isolates from Iran. Exp Parasitol. 2012;130:456–62. doi: 10.1016/j.exppara.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Heidari A, Keshavarz H, Shojaee S, Raeisi A, Dittrich S. In vivo susceptibility of Plasmodium vivax to chloroquine in Southeastern Iran. Iranian J Parasitol. 2012;7:8–14. [PMC free article] [PubMed] [Google Scholar]
- 14.Moon SU, Lee HW, Kim JY, Na BK, Cho SH, Lin K, et al. High frequency of genetic diversity of Plasmodium vivax field isolates in Myanmar. Acta Trop. 2009;109:30–6. doi: 10.1016/j.actatropica.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–52. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 16.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–99. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 17.Nei M, Gojobori T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol. 1986;3:418–26. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- 18.Tajima F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics. 1989;123:585–95. doi: 10.1093/genetics/123.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonald JH, Kreitman M. Adaptive protein evolution at the Adh locus in Drosophila. Nature. 1991;351:652–54. doi: 10.1038/351652a0. [DOI] [PubMed] [Google Scholar]
- 20.Rand DM, Kann LM. Excess amino acid polymorphism in mitochondrial DNA: contrasts among genes form Drosoophila, mice, and humans. Mol Biol Evol. 1996;13:735–48. doi: 10.1093/oxfordjournals.molbev.a025634. [DOI] [PubMed] [Google Scholar]
- 21.Thakur A, Alam MT, Bora H, Kaur P, Sharma YD. Plasmodium vivax: sequence polymorphism and effect of natural selection at apical membrane antigen-1 (PvAMA-1) among Indian population. Gene. 2008;419:35–42. doi: 10.1016/j.gene.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Ord RL, Tami A, Sutherland CJ. AMA-1 genes of sympatric Plasmodium vivax and P. falciparum from Venezuela differ significantly in genetic diversity and recombination frequency. PLoS One. 2008;3:1–9. doi: 10.1371/journal.pone.0003366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zakeri S, Sadeghi H, Mehrizi AA, Djadid ND. Population genetic structure and polymorphism analysis of gene encoding apical membrane antigen-1 (AMA-1) of Iranian Plasmodium vivax wild isolates. Acta Tro. 2013;126:269–79. doi: 10.1016/j.actatropica.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 24.Figtree M, Pasay CJ, Slade R, Cheng Q, Cloonan N, Walker J, Saul A. Plasmodium vivax synonymous substitution frequencies, evolution and population structure deduced from diversity in AMA-1 and MSP-1 genes. Mol Biochem Parasitol. 2000;108:53–66. doi: 10.1016/s0166-6851(00)00204-8. [DOI] [PubMed] [Google Scholar]
- 25.Hughes MK, Hughes AL. Natural selection on Plasmodium surface proteins. Mol. Biochem. Parasitol. 1995;71:99–113. doi: 10.1016/0166-6851(95)00037-2. [DOI] [PubMed] [Google Scholar]
- 26.Escalante AA, Cornejo OE, Rojas A, Udhayakumar V, Lal AA. Assessing the effect of natural selection in malaria parasites. Trends Parasitol. 2004;20:388–95. doi: 10.1016/j.pt.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Polley SD, Chokejindachai W, Conway DJ. Allele frequency-based analyses robustly map sequence site under balancing selection in a malaria vaccine candidate antigen. Genetics. 2003;165:555–61. doi: 10.1093/genetics/165.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polley SD, Conway DJ. Strong diversifying selection on domains of the Plasmodium falciparum apical membrane antigen 1 gene. Genetics. 2001;158:1505–12. doi: 10.1093/genetics/158.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]


