Abstract
Aims: Acoustic startle response in rats is used to model sensorimotor reactivity. The aim of the study was to determine whether acoustic startle response in alcohol-naïve rats predicts subsequent increased voluntary alcohol drinking or alcohol preference. Methods: Startle responses to 90, 95 and 100 decibel (dB) white noise stimuli presented in counterbalanced semi-randomized order were tested in alcohol-naïve young adult male Wistar rats before voluntary alcohol intake was established with an intermittent alcohol access (IAA) model. Results: Startle amplitude in response to 95 or 100 dB stimuli was positively correlated with subsequent alcohol intake and alcohol preference following 3 months of IAA. Rats with high (median split) pre-IAA startle amplitude in response to 95 or 100 dB stimuli developed increased alcohol intake as well as increased alcohol preference following 3 months of IAA, relative to rats with low pre-IAA startle amplitude. Conclusion: Startle response to moderate acoustic stimuli can be a predictive index of vulnerability to developing increased alcohol drinking.
INTRODUCTION
Begleiter and Porjesz (1999) proposed that sensorimotor hyper-reactivity is a key feature of the simplest model of the neuronal milieu underlying a predisposition to alcoholism. This hypothesis is consistent with evidence that sensorimotor hyper-reactivity expressed as enhanced acoustic startle response is characteristic of abstinent alcoholics (Krystal et al., 1997) and is associated with family history of alcoholism (Pfefferbaum et al., 1991; Grillon et al., 1997). Rats selectively bred for alcohol preference and high voluntary alcohol drinking (McKinzie et al., 2000; Chester et al., 2004; Acewicz et al., 2012), and post-dependent rats experiencing either acute alcohol withdrawal or prolonged imposed alcohol abstinence (Rassnick et al., 1992; Rasmussen et al., 2005) also exhibit increased acoustic startle response.
Enhanced startle is associated with increased brain noradrenergic activation (Stevens et al., 1994), and brain noradrenergic activation contributes to increased voluntary alcohol drinking (Walker et al., 2008; Rasmussen et al., 2009; Simpson et al., 2009; Froehlich et al., 2013; O'Neil et al., 2013). Enhanced startle is also correlated with the increased anxiety (Morgan et al., 1993; Davis et al., 1997) that is common to many alcoholics (Cloninger, 1987; Kushner et al., 2000) and that is a major risk factor for alcohol abuse (Koob and Le Moal, 1997). Furthermore, anxiety-related behavior in rats has been demonstrated to predict alcohol drinking under several schedules of alcohol access (Hayton et al., 2012). We thus hypothesized that characterization of startle response may facilitate prospective identification of vulnerability to developing increased voluntary alcohol drinking and also may provide a basis for determining mechanisms mediating development of some alcohol use disorders. Accordingly, we investigated whether prospectively determined acoustic startle response in alcohol-naïve rats was correlated with subsequent increased voluntary alcohol drinking or increased alcohol preference in an intermittent alcohol access (IAA) model. IAA, in which rats have access to 2-bottle choice (water vs 20% alcohol) home cage alcohol drinking for three 24-h sessions/week, separated by at least 24 h (e.g. Monday, Wednesday, Friday), has been reported to induce outbred Wistar rats to escalate alcohol intake over repetitive access sessions to achieve alcohol intake at individually variable high levels accompanied by high alcohol preference and blood alcohol concentrations (BACs) comparable to those achieved by selectively bred alcohol-preferring (P) rats, and has been suggested to effectively model some human alcohol use disorders (Wise, 1973; Simms et al., 2008).
MATERIALS AND METHODS
Animals
Twenty-three alcohol-naïve young adult male Wistar rats (Simonsen Labs, Gilroy, CA, USA) weighing 285 ± 3 g were housed 2/cage in plastic shoebox cages with controlled temperature (21 ± 1°C) and a 12 h/12 h light/dark cycle (lights off at 0900 h). Standard rodent chow (Laboratory Rodent Diet #7001, Harlan Teklad, Madison, WI, USA) and water were available ad libitum throughout the study. All experimental procedures were approved by the Veterans Administration Puget Sound Health Care System Institutional Animal Care and Use Committee and conducted in compliance with the NIH Guide for the Care and Use of Laboratory Animals.
Acoustic startle testing system
Acoustic startle was tested with an SR-LAB Acoustic Startle System (SDI, San Diego, CA, USA) using a slight modification of methods we previously reported (Rasmussen et al., 2008). Each SR-LAB test chamber includes a ventilated sound-attenuated cabinet containing a clear plastic cylindrical rat enclosure mounted on a piezoelectric accelerometer that detects muscle twitch in response to a brief pulse of white (mixed frequency) noise produced by a tweeter inside the cabinet. The force exerted on the accelerometer is digitized and expressed in millivolt (mV) units. Each startle response signal generated by the accelerometer was recorded as 65 consecutive 1 ms recordings, starting at the onset of each 40-ms startle tone. Results were analyzed as maximum peak amplitude. Startle stimulus and background white noise levels were calibrated with a Radio Shack Digital Sound Level Meter (33–2055; RadioShack Corp., Fort Worth, TX, USA) placed in the center of the cylindrical rat enclosure. Each of 8 SR-LAB test chambers was calibrated before each use to provide 450 mV response to a consistent test stimulus provided by an SDI Standardization Unit (SDI, San Diego, CA, USA). Each SR-LAB test chamber and rat enclosure was cleaned with 0.5% Liquinox (Alconox, Inc., New York, NY, USA) before each use.
Pre-test acclimation
After acclimation to the animal colony room and the reversed light/dark cycle for 3 weeks, rats were transferred to a dark testing room adjoining the colony room for 90 min at 1.5–3 h after lights-off on each of 5 days prior to testing, with ambient 60 decibel (dB) white noise produced by a White Noise Generator (SDI, San Diego, CA, USA). On the first 4 days, each rat was acclimated to a dark SR-LAB test chamber for 5 min with 60 dB white background noise (produced by the tweeter inside the chamber) before being returned to the colony room, housed with the same cage mate. On the fifth day, each of the rats was likewise transferred to the dark testing room with ambient 60 dB white noise, placed in the dark SR-LAB test chamber for 5 min with 60 dB white background noise, and then exposed to 10 presentations of 40 ms 95 dB white noise pulses presented at 30 s intervals before being returned to the colony room. The goal of this initial exposure to acoustic pulses was to minimize effects of novelty stress in the subsequent acoustic startle testing with similar white noise pulses. Immediately following completion of the acclimation process, each rat was returned to the same colony room but individually housed in a plastic shoebox cage. All procedures during the acclimation and subsequent acoustic startle testing and IAA were conducted under dim red illumination.
Acoustic startle testing
Startle testing was conducted 7–10 days after completion of the pre-test acclimation. On the test day, rats were again transferred to the dark testing room with 60 dB background white noise. After 90 min, each rat was placed in a dark SR-LAB testing chamber with 60 dB background white noise for 5 min before quantitation of startle responses to 10 presentations each of 40 ms 90, 95 or 100 dB white noise pulses at 30 s intervals, with one pulse of each intensity (i.e. 90, 95 or 100 dB) in counterbalanced order within each of 10 sequential sets of three pulses (Table 1), with 60 dB background white noise between pulses. Thus, there were a total of 30 startle tests at 30 s intervals, with 10 tests/each of responses to 90, 95 or 100 dB pulses distributed in counterbalanced order over a 15-min period.
Table 1.
Order of acoustic stimuli presentations
| Sequential sets of 3 acoustic stimuli | Counterbalanced order of stimuli within sets (dB) |
||
|---|---|---|---|
| 1 | 90 | 95 | 100 |
| 2 | 95 | 100 | 90 |
| 3 | 100 | 90 | 95 |
| 4 | 90 | 95 | 100 |
| 5 | 95 | 100 | 90 |
| 6 | 100 | 90 | 95 |
| 7 | 90 | 95 | 100 |
| 8 | 95 | 100 | 90 |
| 9 | 100 | 90 | 95 |
| 10 | 90 | 95 | 100 |
There were 30 s intervals between stimuli within each set as well as between each sequential set.
Alcohol drinking
Three weeks after startle testing, 2-bottle choice access to 20% (v/v) alcohol vs water was provided for 24 h/day, 3 days/week (M, W, F)—i.e. an IAA model (Wise, 1973; Simms et al., 2008). The alcohol solution was prepared by diluting 95% alcohol (ethanol; Decon Labs, King of Prussia, PA, USA) with deionized water to make a 20% (v/v) solution. Alcohol (20%) and water were presented in ball-bearing sipper tubes, with positions of the tubes alternated in sequential alcohol access periods to control for potential side preferences. On days when alcohol was not provided, the rats had access to water only. On days when alcohol and water intakes were characterized for analysis, daily fluid intakes were determined by weighing each tube to the nearest 0.1 g. Alcohol and water tubes were also placed on two empty cages to determine loss due to spillage/leakage and evaporation; average losses in these two cages on each day were subtracted from intakes for that day. Net daily alcohol intake was converted to g alcohol/kg body weight. After 36 alcohol access days (i.e. 12 weeks, when stable alcohol intake was achieved) alcohol intake and alcohol preference (ml of alcohol intake/[ml of alcohol intake + ml of water intake]) were determined over the next 3 alcohol access days to characterize the relationships of each rat's alcohol intake and alcohol preference relative to its pre-IAA acoustic startle responses. One rat did not establish significant daily alcohol drinking (alcohol intake was <1 g/kg/day on all days evaluated) and was excluded from further analyses.
Data analyses
Startle amplitude in response to presentations of 90, 95 or 100 dB acoustic pulse intensities in counterbalanced order within each of 10 sequential sets of 3 pulse presentations were initially evaluated by two-way (set X pulse intensity) repeated measures analysis of variance (ANOVA) with repeated measures on sets (1–10) and pulse intensities (90, 95 or 100 dB). There was a significant effect of intensity, F(2, 42) = 63.7, P < 0.001, but no significant effect of set and no significant intensity × set interaction. Since startle amplitude in response to each of the acoustic stimulus intensities was independent of presentation time (set) within the 15 min test period, the average of all 10 responses to each stimulus intensity was used in subsequent analyses of the relationships between pre-IAA acoustic startle response vs IAA alcohol intake or alcohol preference. Similarly, IAA alcohol intake or alcohol preference on the 3 alcohol access days in IAA week 13 was analyzed by one-way ANOVA with repeated measures on day; there were no significant effects of day on either alcohol intake or alcohol preference, so 3-day average alcohol intake or 3-day average alcohol preference was likewise used in subsequent analyses of relationships between IAA week 13 alcohol intake or alcohol preference vs pre-IAA acoustic startle amplitude.
Pre-IAA startle amplitude in response to presentations of either 90, 95 or 100 dB stimuli was each compared with subsequent alcohol intake or alcohol preference by Pearson Product Moment Correlation Analysis. The pre-IAA startle response to 90, 95 or 100 dB stimuli in rats grouped on the basis of high vs low (median split, n = 11/group) IAA week 13 alcohol intake was further compared by two-way (high vs low alcohol intake X stimulus intensity) ANOVA with repeated measures on stimulus intensity (90, 95, 100 dB). The pre-IAA startle amplitude in response to 90, 95 or 100 dB stimuli in rats grouped on the basis of high vs low (median split, n = 11/group) IAA week 13 alcohol preference was likewise compared by two-way (high vs low alcohol preference X stimulus intensity) ANOVA with repeated measures on stimulus intensity (90, 95, 100 dB). The IAA week 13 alcohol intake or alcohol preference of rats grouped on the basis of high vs low (median split, n = 11/group) pre-IAA startle amplitude in response to either 90, 95 or 100 dB stimuli was each analyzed by Student t-test (median splits of startle responses to each of the three stimulus intensities did not in each case identify the same animals to be included in the high vs low startle response groups, so two-way ANOVA testing could not be performed); Bonferroni corrections were not applied to individual t-tests.
All analyses were conducted using Sigmaplot Version 11 software (Systat Software, Inc., Chicago, IL, USA) with significance accepted at P < 0.05. Data are presented as mean ± SEM.
RESULTS
IAA alcohol intake and alcohol preference
Initial (i.e. IAA week 1) average (M, W, F) alcohol intake was 0.95 ± 0.16 g/kg/24 h and alcohol preference was 0.07 ± 0.01. By IAA week 13, alcohol intake had increased to 3.80 ± 0.32 g/kg/24 h (P < 0.001) and alcohol preference had increased to 0.40 ± 0.03 (P < 0.001).
IAA alcohol intake relative to pre-IAA startle response
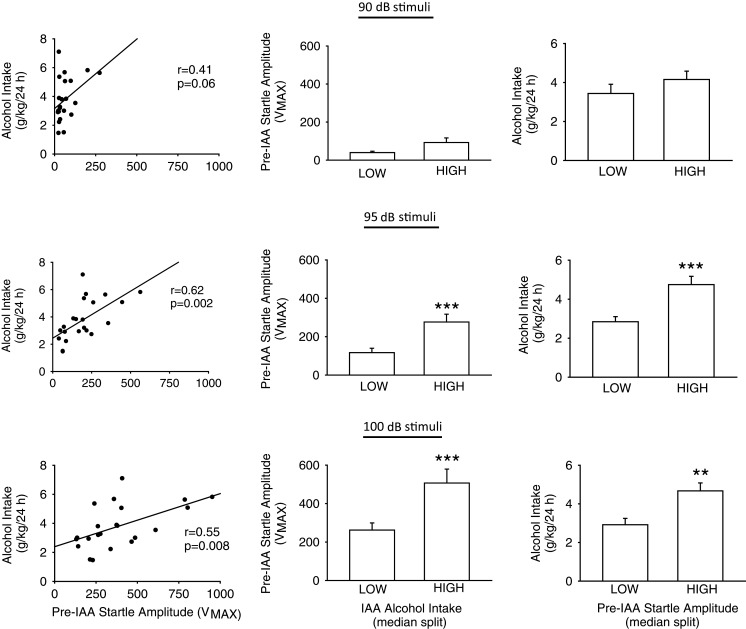
IAA week 1 alcohol intake was not significantly correlated with pre-IAA startle amplitude elicited in response to 90 (P = 0.18), 95 (P = 0.11) or 100 (P = 0.15) dB stimuli. IAA week 13 alcohol intake relative to pre-IAA startle amplitude elicited in response to presentations of either 90, 95 or 100 dB stimuli is presented in the upper, middle or lower row, respectively, of Fig. 1.
Fig. 1.
Pre-IAA acoustic startle response vs alcohol intake following 3 months of IAA. Rows: The upper, middle and lower rows present analyses of pre-IAA responses to 90, 95 or 100 dB acoustic startle stimuli, respectively. Columns: The left panel in each row presents the correlation between pre-IAA acoustic startle amplitude vs IAA week 13 alcohol intake for all 22 rats. The center panel in each row presents the pre-IAA acoustic startle amplitude of rats grouped on the basis of low vs high (median split, n = 11 rats/group) alcohol intake in IAA week 13. The right panel in each row presents the IAA week 13 alcohol intake of rats grouped on the basis of low vs high (median split, n = 11 rats/group) pre-IAA acoustic startle amplitude. **P ≤ 0.01 vs Low, ***P ≤ 0.001 vs Low.
Pre-IAA startle amplitude in response to 90 dB stimuli was modest and inconsistent; startle amplitude was not significantly correlated with alcohol intake established by 3 subsequent months of IAA (Fig. 1, upper row, left). Grouping the rats on the basis of high vs low (median split) IAA week 13 alcohol intake revealed no alcohol intake-dependent significant difference in pre-IAA startle in response to 90 dB stimuli (Fig. 1, upper row, center; in the two-way ANOVA with repeated measures on stimulus intensity, there was a significant overall [alcohol intake (high, low) × stimulus intensity (90, 95,100 dB)] interaction, F[2, 40] = 5.1, P ≤ 0.01, but pre-IAA startle amplitude in response to the 90 dB stimulus was not significantly different between the high and low alcohol intake groups). Grouping on the basis of high vs low (median split) pre-IAA startle response to 90 dB stimuli likewise revealed no pre-IAA startle amplitude-dependent significant difference in IAA week 13 alcohol intake (Fig. 1, upper row, right).
Pre-IAA startle amplitude in response to 95 dB stimuli was positively correlated with alcohol intake established by 3 subsequent months of IAA (Fig. 1, middle row, left; P < 0.01, r = 0.62). Rats with high (median split) alcohol intake in IAA week 13 had previously exhibited greater pre-IAA startle response to 95 dB stimuli, relative to rats with low alcohol intake in IAA week 13 (Fig. 1, middle row, center; P < 0.001). Consistent with this result, rats with high (median split) pre-IAA startle amplitude in response to 95 dB stimuli subsequently developed increased alcohol intake in IAA week 13, relative to rats with low pre-IAA startle response to 95 dB stimuli (Fig. 1, middle row, right; P ≤ 0.001).
Pre-IAA startle amplitude in response to 100 dB stimuli also was positively correlated with alcohol intake established by 3 subsequent months of IAA (Fig. 1, lower row, left; P < 0.01, r = 0.55). Rats with high (median split) alcohol intake in IAA week 13 had previously exhibited greater pre-IAA startle response to 100 dB stimuli (Fig. 1, lower row, center; P < 0.001). Consistent with this results, rats with high (median split) pre-IAA startle response to 100 dB stimuli subsequently developed increased alcohol intake in IAA week 13, relative to rats with low pre-IAA startle response to 100 dB stimuli (Fig. 1, lower row, right; P < 0.01).
IAA alcohol preference relative to pre-IAA startle response
IAA week 1 alcohol preference was not significantly correlated with pre-IAA startle amplitude elicited in response to 90 (P = 0.19), 95 (P = 0.14) or 100 (P = 0.20) dB stimuli.
IAA week 13 alcohol intake and alcohol preference were highly positively correlated, r = 0.92, P < 0.001. Further analyses of IAA week 13 alcohol preference relationships to pre-IAA startle responses were conducted identically to those in the preceding analysis of alcohol intake relationships to pre-IAA startle responses. Consistent with the high positive correlation between alcohol intake and alcohol preference, the results of analyses of alcohol preference vs pre-IAA startle responses, as detailed below, were essentially identical to the results of the preceding analyses of alcohol intake vs pre-IAA startle responses. Pre-IAA startle amplitude in response to 90 dB stimuli was positively correlated with alcohol preference established by 3 subsequent months of IAA (P < 0.05, r = 0.47). Grouping the rats on the basis of high vs low (median split) IAA week 13 alcohol preference revealed no alcohol preference-dependent significant difference in pre-IAA startle response to 90 dB stimuli (in the two-way ANOVA with repeated measures on stimulus intensity, there was a significant overall [alcohol intake (high, low) × stimulus intensity (90, 95, 100 dB)] interaction, F[2, 40] = 6.41, P < 0.01, but pre-IAA startle in response to the 90 dB stimulus was not significantly different between the high and low alcohol intake groups). Grouping on the basis of high vs low (median split) pre-IAA startle response to 90 dB stimuli likewise revealed no pre-IAA startle amplitude-dependent significant difference in IAA week 13 alcohol preference.
Pre-IAA startle amplitude in response to 95 dB pulses was positively correlated with alcohol preference established by 3 subsequent months of IAA (P < 0.01, r = 0.57). Rats with high (median split) alcohol preference in IAA week 13 had previously exhibited greater pre-IAA startle response to 95 dB stimuli, relative to rats with low alcohol preference in IAA week 13 (P < 0.05). Consistent with this result, rats with high (median split) pre-IAA startle response to 95 dB stimuli subsequently developed increased alcohol preference in IAA week 13, relative to rats with low pre-IAA low startle response to 95 dB stimuli (P < 0.01).
Pre-IAA startle response to 100 dB stimuli also was positively correlated with alcohol preference established by 3 subsequent months of IAA (P < 0.01, r = 0.53). Rats with high (median split) alcohol preference in IAA week 13 had previously exhibited greater pre-IAA startle response to 100 dB stimuli, relative to rats with low alcohol preference in IAA week 13 (P < 0.001). Consistent with this result, rats with high (median split) pre-IAA startle response to 100 dB stimuli subsequently developed increased alcohol preference in IAA week 13, relative to rats with low pre-IAA startle response to 100 dB stimuli (P < 0.01).
IAA alcohol intake relative to pre-IAA startle response to the first presentation of each stimulus intensity
Some previous investigations of alcohol drinking in rats have compared alcohol intake or preference relative to startle amplitude in response to only the first presentation of an acoustic stimulus. In the current study, IAA week 13 alcohol intake or alcohol preference was not significantly correlated with pre-IAA startle in response to the first presentation of either 90 or 100 dB stimuli. However, pre-IAA startle amplitude in response to the first 95 dB stimulus was correlated with IAA week 13 alcohol intake (r = 0.66; P ≤ 0.001) as well as alcohol preference (r = 0.56; P < 0.01).
DISCUSSION
In alcohol-naïve young adult male Wistar rats, acoustic startle amplitude in response to 40 ms pulses of white noise at intensities of 95 or 100 dB was positively correlated with subsequent voluntary alcohol intake and alcohol preference following 3 months of IAA. Rats with high (median split) alcohol intake or alcohol preference following the 3 months of IAA had previously exhibited greater pre-IAA startle response to 95 as well as 100 dB stimuli, relative to rats with low alcohol intake or low alcohol preference. Conversely, rats with high (median split) pre-IAA startle response to 95 or 100 dB stimuli subsequently developed increased alcohol intake as well as increased alcohol preference following 3 months of IAA.
Stimulus intensities in this investigation were based on the results of preliminary trials with young male Wistar rats in which 90 dB stimuli produced inconsistent small startle responses, 95 or 100 dB stimuli reliably produced relatively consistent sub-maximal startle, and a higher intensity stimulus (120 dB) produced maximal responses. The moderate 90, 95 and 100 dB stimuli were selected in order to avoid ceiling effects that could compromise ability to differentiate responses between animals, as suggested by a report that human startle amplitudes elicited by 90 dB, but not 114 dB, stimuli were positively correlated with number of previous alcohol detoxifications (Krystal et al., 1997). It previously has been reported that male Wistar rats exhibited an inverted U-shaped curvilinear relationship between the startle response to an initial 120 dB acoustic stimulus vs later alcohol intake, and that startle habituation appeared to have predictive value regarding alcohol intake (Sandbak et al., 2000). In the current study, habituation to repeated stimulus exposures was not apparent, and there were significant positive linear correlations between pre-IAA acoustic startle responses to 95 or 100 dB stimuli vs alcohol intake and alcohol preference following IAA. The apparent disparities between the Sandbak et al. (2000) study and the current study may be due to the differing stimulus intensities as well as to the incorporation of an initial session with exposure to repetitive moderate (95 dB) stimuli in advance of the testing trial in the current study in order to minimize novelty of the stimulus (consistent with clinical studies, in which the subjects are aware that they will hear acoustic stimuli during the testing trial). In addition, stimuli of three different intensities were presented in semi-random counterbalanced order throughout the 15 min trial in the current study, rather than consistent repetition of a single stimulus. It is also notable that the current study used an IAA model of alcohol drinking in which a relatively high concentration of alcohol (20%, v/v) was available on 3 intermittent days each week, considered to be a model for excessive alcohol drinking (Wise, 1973; Simms et al., 2008).
Alcohol-naïve rats from lines selectively bred to prefer alcohol exhibit increased acoustic startle relative to selectively bred alcohol non-preferring rats (McKinzie et al., 2000; Chester et al., 2004; Acewicz et al., 2012). Sons of alcoholics likewise exhibited increased acoustic startle compared with sons of non-alcoholic parents (Grillon et al., 1997). The current results suggest that mechanisms contributing to acoustic startle response have a functional role in the vulnerability to increased voluntary alcohol drinking, and that acoustic startle characterization can provide an index of sensorimotor hyper-reactivity and associated mechanisms that contribute to this increased alcohol drinking. Although these mechanisms remain to be resolved, it has been demonstrated that brain noradrenergic activation increases acoustic startle response (Stevens et al., 1994) and also produces sensorimotor hyper-reactivity and anxiety (Redmoond and Huang, 1979; Sullivan et al., 1999) which are major risk factors for development of alcohol use disorders (Cloninger, 1987; Koob and Le Moal, 1997; Begleiter and Porjesz, 1999; Kushner et al., 2000). Conversely, suppression of noradrenergic signaling not only decreases acoustic startle responses (Gresack and Risbrough, 2011; Olson et al., 2011) but also decreases alcohol drinking in rats and humans (Walker et al., 2008; Rasmussen et al., 2009; Simpson et al., 2009; Froehlich et al., 2013; O'Neil et al., 2013) and blocks the expression of increased alcohol drinking in rats selectively bred for alcohol intake (Froehlich et al., 2013). The consistent association of changes in acoustic startle, anxiety and increased alcohol drinking with changes in noradrenergic signaling suggests that noradrenergic activation may have a key role in mediating the correlation between acoustic startle amplitude and subsequent development of increased voluntary alcohol drinking.
The current results demonstrate that acoustic startle amplitude in response to moderately supra-threshold startle stimulus intensities administered to alcohol-naïve male Wistar rats is an effective predictive index for subsequent increased voluntary alcohol intake and alcohol preference in the IAA model. Acoustic startle response may be an especially useful index of the vulnerability to developing increased alcohol drinking because it is not dependent upon, and potentially confounded by, interactions with other behaviors. Importantly, acoustic startle is also well-characterized for use in humans (Krystal et al., 1997; Grillon and Baas, 2003; Grillon et al., 1998, 2005), providing translational utility.
These results may provide a useful model for investigating neurobiological mechanisms mediating initiation and development of excessive alcohol drinking, as well as provide the conceptual basis for a potential approach to prospectively identifying individuals at increased risk for future alcohol use disorders, thus allowing potential preventive intervention.
Funding
This work was supported in part by resources from the VA Puget Sound Health Care System, Seattle, Washington; US Army Medical Research Acquisition Activity CDMRP (contract number W81XWH-13-1-0126); and National Institutes of Health (grant number AA10567).
Conflict of interest statement
None declared.
REFERENCES
- Acewicz A, Mierzejewski P, Jastrzebska A, et al. Acoustic startle responses and prepulse inhibition of acoustic startle responses in Warsaw alcohol high-preferring (WHP) and Warsaw alcohol low-preferring (WLP) rats. Alcohol Alcohol. 2012;47:386–9. doi: 10.1093/alcalc/ags039. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. What is inherited in the predisposition toward alcoholism? A proposed model. Alcohol Clin Exp Res. 1999;23:1125–35. doi: 10.1111/j.1530-0277.1999.tb04269.x. [DOI] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Froehlich JC. Acoustic startle reactivity during acute alcohol withdrawal in rats that differ in genetic predisposition toward alcohol drinking: Effect of stimulus characteristics. Alcohol Clin Exp Res. 2004;28:677–87. doi: 10.1097/01.alc.0000125345.19665.09. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–6. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Roles of the amygdala and bed nucleus of the stria terminalis in fear and anxiety measured with the acoustic startle reflex. Possible relevance to PTSD. Ann N Y Acad Sci. 1997;821:305–31. doi: 10.1111/j.1749-6632.1997.tb48289.x. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer BJ, Federoff DL, et al. Prazosin reduces alcohol drinking throughout prolonged treatment and blocks the initiation of drinking in rats selectively bred for alcohol intake. Alcohol Clin Exp Res. 2013;37:1552–60. doi: 10.1111/acer.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Risbrough VB. Corticotropin-releasing factor and noradrenergic signaling exert reciprocal control over startle reactivity. Int J Neuropsychopharmacol. 2011;14:1179–94. doi: 10.1017/S1461145710001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol. 2003;114:1557–79. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Dierker L, Merikangas KR. Startle modulation in children at risk for anxiety disorder and/or alcoholism. J Am Acad Child Adolesc Psychiatry. 1997;36:925–32. doi: 10.1097/00004583-199707000-00014. [DOI] [PubMed] [Google Scholar]
- Grillon C, Dierker L, Merikangas KR. Fear-potentiated startle in adolescent offspring of parents with anxiety disorders. Biol Psychiatry. 1998;44:990–7. doi: 10.1016/s0006-3223(98)00188-7. [DOI] [PubMed] [Google Scholar]
- Grillon C, Warner V, Hille J, et al. Families at high and low risk for depression: A three-generation startle study. Biol Psychiatry. 2005;57:953–60. doi: 10.1016/j.biopsych.2005.01.045. [DOI] [PubMed] [Google Scholar]
- Hayton SJ, Mahoney MK, Olmstead MC. Behavioral traits predicting alcohol drinking in outbred rats: an investigation of anxiety, novelty seeking, and cognitive flexibility. Alcohol Clin Exp Res. 2012;36:594–603. doi: 10.1111/j.1530-0277.2011.01668.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–8. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Webb E, Grillon C, et al. Evidence of acoustic startle hyperreflexia in recently detoxified early onset male alcoholics: Modulation by yohimbine and m-chlorphenylpiperazine (mCPP) Psychopharmacology. 1997;131:207–15. doi: 10.1007/s002130050285. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev. 2000;20:149–71. doi: 10.1016/s0272-7358(99)00027-6. [DOI] [PubMed] [Google Scholar]
- McKinzie DL, Sajdyk TJ, McBride WJ, et al. Acoustic startle and fear-potentiated startle in alcohol-preferring (P) and -nonpreferring (NP) lines of rats. Pharmacol Biochem Behav. 2000;65:691–6. doi: 10.1016/s0091-3057(99)00252-x. [DOI] [PubMed] [Google Scholar]
- Morgan CAI, Southwick SM, Grillon C, et al. Yohimbine-facilitated acoustic startle reflex in humans. Psychopharmacology. 1993;110:342–6. doi: 10.1007/BF02251291. [DOI] [PubMed] [Google Scholar]
- Olson VG, Rockett HR, Reh RK, et al. The role of norepinephrine in differential response to stress in an animal model of posttraumatic stress disorder. Biol Psychiatry. 2011;70:441–8. doi: 10.1016/j.biopsych.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil ML, Beckwith LE, Kincaid CL, et al. The α1-adrenergic receptor antagonist, doxazosin, reduces alcohol drinking in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2013;37:202–12. doi: 10.1111/j.1530-0277.2012.01884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, White PM, et al. Event-related potentials in alcoholic men: P3 amplitude reflects family history but not alcohol consumption. Alcohol Clin Exp Res. 1991;15:839–50. doi: 10.1111/j.1530-0277.1991.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Burke B, Crites NJ. Chronic daily ethanol and withdrawal: melatonin treatment reverses persistently increased acoustic startle response during abstinence. Alcohol Clin Exp Res. 2005;29(Suppl):16A. [Google Scholar]
- Rasmussen DD, Crites NJ, Burke BL. Acoustic startle amplitude predicts vulnerability to develop post-traumatic stress hyper-responsivity and associated plasma corticosterone changes in rats. Psychoneuroendocrinology. 2008;33:282–91. doi: 10.1016/j.psyneuen.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Alexander LL, Raskind MA, et al. The α1-adrenergic receptor antagonist, prazosin, reduces alcohol drinking in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2009;33:264–72. doi: 10.1111/j.1530-0277.2008.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassnick S, Koob GF, Geyer MA. Responding to acoustic startle during chronic ethanol intoxication and withdrawal. Psychopharmacology. 1992;106:351–8. doi: 10.1007/BF02245417. [DOI] [PubMed] [Google Scholar]
- Redmoond DEJ, Huang YH. Current concepts. II. New evidence for a locus coeruleus-norepinephrine connection with anxiety. Life Sci. 1979;25:2149–62. doi: 10.1016/0024-3205(79)90087-0. [DOI] [PubMed] [Google Scholar]
- Sandbak T, Rimol LM, Jellestad FK, et al. Relating acoustic startle reactivity and plasticity to alcohol consumption in male Wistar rats. Physiol Behav. 2000;68:723–33. doi: 10.1016/s0031-9384(99)00239-5. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, et al. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–23. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TL, Saxon AJ, Meredith CW, et al. A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcohol Clin Exp Res. 2009;33:255–63. doi: 10.1111/j.1530-0277.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Coplan JD, Kent JM, et al. The noradrenergic system in pathological anxiety: a focus on panic with relevance to generalized anxiety and phobias. Biol Psychiatry. 1999;46:1205–18. doi: 10.1016/s0006-3223(99)00246-2. [DOI] [PubMed] [Google Scholar]
- Stevens DR, McCarley RW, Greene RW. The mechanism of noradrenergic alpha-1 excitatory modulation of pontine reticular formation neurons. J Neurosci. 1994;14:6481–7. doi: 10.1523/JNEUROSCI.14-11-06481.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Rasmussen DD, Raskind MA, et al. The effects of α1-noradrenergic receptor antagonism on dependence-induced increases in responding for ethanol. Alcohol. 2008;42:91–7. doi: 10.1016/j.alcohol.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–10. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]