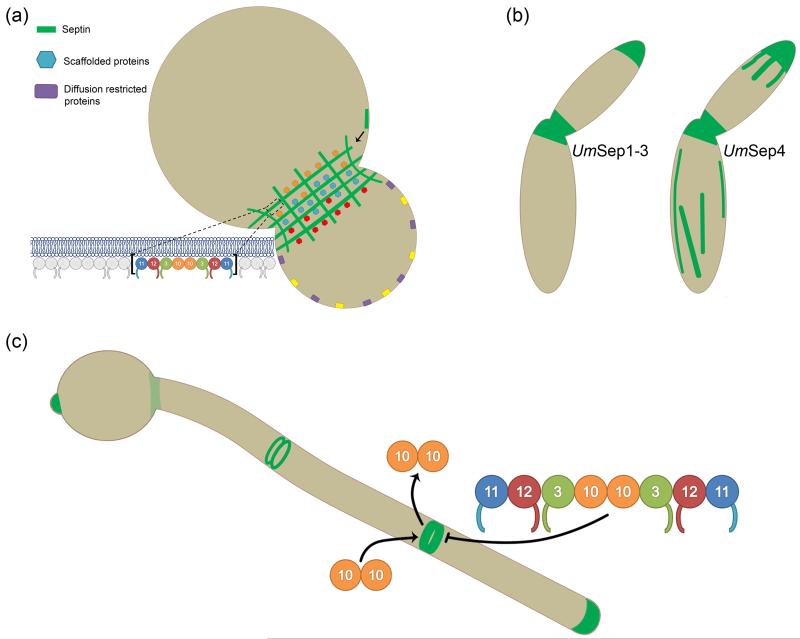
Figure 1.
Septin localization and dynamics. (a) In S. cerevisiae, septins localize to the bud-neck plasma membrane in a gauze-like arrangement of filaments that act as molecular scaffolds for many proteins, some of which preferentially localize to one side of the bud-neck [4]. Septin higher-order structures assemble from palindromic rod subunits via a process involving filament annealing and once assembled are capable of restricting membrane associated proteins to one side of the cell [3,22]. (b) In the plant pathogen U. maydis, septins Sep1, Sep2, and Sep3 localize to the cell middle and at growing tips [30]. Notably, Sep4 also localizes at these sites but additionally forms fibers structures running throughout the cell, which partially colocalize with, but do not depend on, microtubules [30]. (c) Though all septins colocalize in C. albicans hyphae, their dynamics are separable. While Cdc3, Cdc11, Cdc12 and Shs1 assembled into higher order structures do not readily exchange with cytoplasmic septins as determined by FRAP, Cdc10 does. Interestingly, Cdc10, the only septin lacking a c-terminal coiled-coil domain, was found to recover after photobleaching only when Shs1 was not mutated [29].