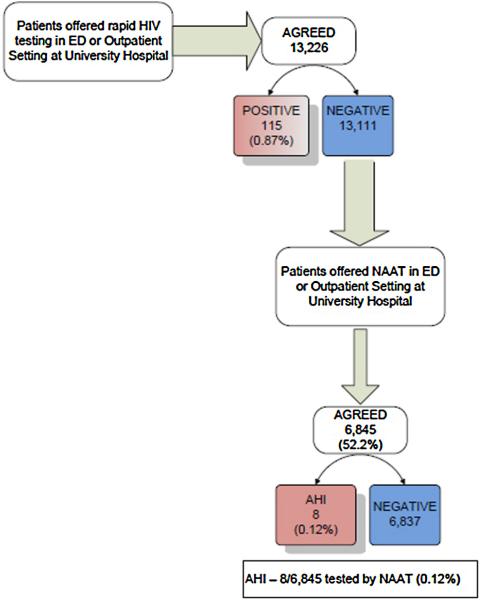
Fig. 1.
Testing flowchart – patients were approached in the Emergency Department and in an Outpatient Clinic at University Hospital, Newark, NJ and offered rapid HIV screening. After rapid HIV screening, using the StatPak Rapid HIV 1/2 test (Alere North America, Inc., Princeton, NJ), those determined to be negative (13,111) were offered an opportunity to participate in pooled nucleic acid amplification testing (NAAT). Plasma samples were collected, centrifuged and stored frozen from those agreeing (6845) until a 27 sample batch could be pooled and transported to the University of Washington, Center for AIDS Research (CFAR) for analysis.