Figure 6. IB2-mediated p38δ activation.
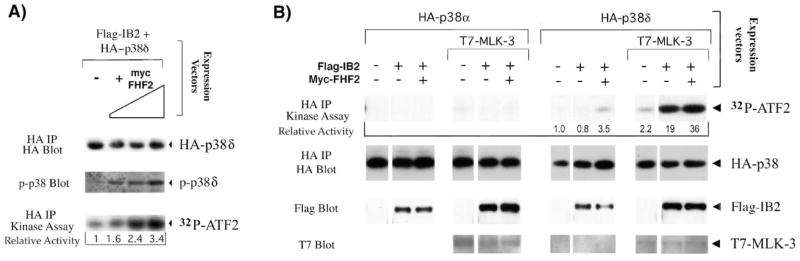
A) FHF2 induces activation of p38δ. 293T cells were transfected with plasmids expressing HA-p38δ, Flag-tagged IB2 and increasing amounts of Myc-tagged FHF2 and serum starved for 5 hours before cell lysis. HA-p38δ activity in extracts was assayed by probing Western blots of electrophoresed extracts with antibodies specific for dually phosphorylated p38. Alternatively, HA-p38δ activity was assayed by its ability to phosphorylate GST-ATF-2 in kinase assays as described in Figure 2A. 32P-ATF-2 levels were visualized by autoradiography and analyzed using PhosphorImager imaging and ImageQuant software, and recalculated as activity relative to the activity of each kinase in the absence of FHF2. Western blot probing with anti-HA after anti-HA immunoprecipitation was used to detect the amount of HA-p38δ proteins assayed in the kinase assay. Extracts analyzed in this panel have also been used to demonstrate FHF-2-mediated recruitment of p38δ to IB2 (26). B) Synergistic activation of p38δ by MLK-3 and IB2 is stimulated by FHF. 293T cells were transfected with plasmids expressing HA-p38δ or HA-p38δ, Flag-tagged IB2, T7-tagged MLK-3 and Myc-tagged FHF2 in different combinations as indicated and serum starved for 5 hours. HA-p38δ activity in extracts was assayed in kinase assays as described in the legend to Figure 2A. 32P incorporation into ATF-2 was visualized and analyzed as described above and recalculated as activity relative to the activity of each kinase in the absence of other transfected proteins to determine the relative p38 activities (kinase activity in absence of cotransfected proteins = 1.0). The same blot was probed with anti-HA to detect HA-p38 proteins. Western blots of total cell lysates were probed with anti-Flag or anti-T7 to detect IB2 and MLK-3 proteins, respectively.
