Figure 2. Inflammasome activation in atherosclerosis and type 2 diabetes.
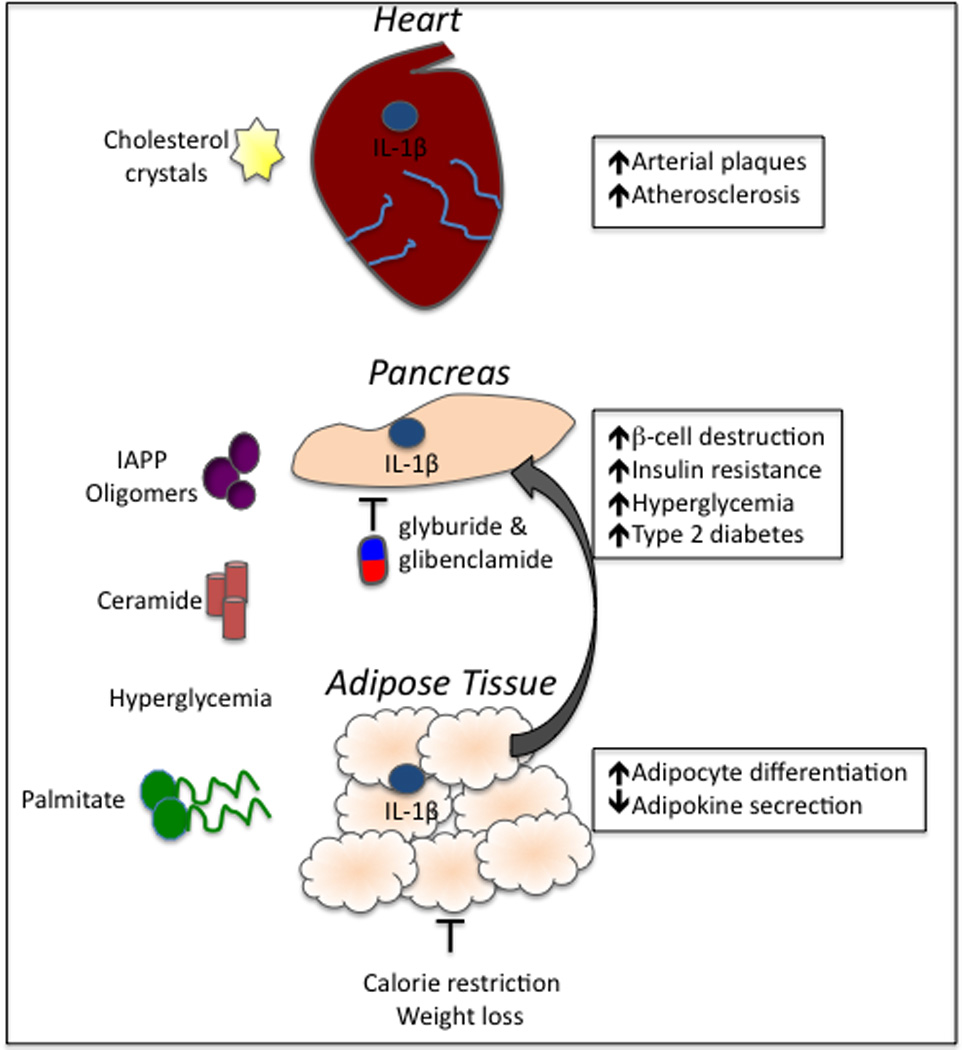
Obesity-related factors have emerged as important activators of inflammasome-derived cytokines. Cholesterol crystals that develop as a result of high-fat diets and obesity deposit in the arterial walls and incite inflammation via the activation of the NLRP3 inflammasome. Unrestrained production of IL-1β and IL-18 induces the production of additional inflammatory cytokines and provokes the recruitment of immune cells, including macrophages. Inflammasome activation in the cardiovascular system can ultimately result in plaque rupture. Obesity-derived metabolites, including IAAP oligomers and fatty acids (palmitate and ceramide), can induce inflammasome activation, which contributes to the induction and progression of T2D. Furthermore, hyperglycemia and alteration of adipose tissue causes metabolic distress and direct downstream activation of inflammasome signaling. Secretion of IL-1β and IL-18 in the pancreas causes the destruction of insulin producing cells (β-cells) and hyperglycemia. Inflammasome-mediated inflammation in the adipose tissue impairs adipokine production, which is an important regulator of insulin-sensitization. Two commonly prescribed T2D drugs, glyburide and glibenclamide, limit disease partially through their inhibition of inflammasome activation. Weight loss can also limit the secretion of inflammasome-induced cytokines.
