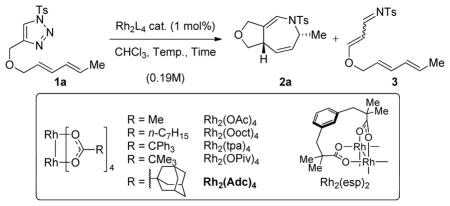
Table 1.
Catalyst optimization for the intramolecular Rh(II)-catalyzed dihydroazepine formation[a]
| ||||
|---|---|---|---|---|
| Entry | Rh2L4 | Temp.(°C) | Time | Yield 2a(%)[b],[c] |
| 1 | Rh2(Ooct)4 | 140[d] | 0.25 h | 54 (34) |
| 2 | Rh2(OAc)4 | 140[d] | 0.25 h | 47 (48) |
| 3 | Rh2(tpa)4 | 140[d] | 0.25 h | 18 (70) |
| 4 | Rh2(OPiv)4 | 140[d] | 0.25 h | 58 (18) |
| 5 | Rh2(esp)2 | 140[d] | 0.25 h | 66 (33) |
| 6 | Rh2(Adc)4 | 140[d] | 0.25 h | 68 (17) |
| 7 | Rh2(Adc)4 | 60 | 16 h | 74 (14) |
Only one diastereomer of the dihydroazepine product 2a was observed by 1H NMR in all cases.
Isolated yield.
NMR yield of imine 3 in parentheses.
Reaction was performed in a microwave apparatus.
