Abstract
Background
Accurate antenna placement is essential for effective microwave ablation (MWA) of lesions. Laparoscopic targeting is made particularly challenging in liver tumours by the needle's trajectory as it passes through the abdominal wall into the liver. Previous optical three-dimensional guidance systems employing infrared technology have been limited by interference with the line of sight during procedures.
Objective
The aim of this study was to evaluate a newly developed magnetic guidance system for laparoscopic MWA of liver tumours in a pilot study.
Methods
Thirteen patients undergoing laparoscopic MWA of liver tumours gave consent to their participation in the study and were enrolled. Lesion targeting was performed using the InnerOptic AIM™ 3-D guidance system to track the real-time position and orientation of the antenna and ultrasound probe.
Results
A total of 45 ablations were performed on 34 lesions. The median number of lesions per patient was two. The mean ± standard deviation lesion diameter was 18.0 ± 9.2 mm and the mean time to target acquisition was 3.5 min. The first-attempt success rate was 93%. There were no intraoperative or immediate postoperative complications. Over an average follow-up of 7.8 months, one patient was noted to have had an incomplete ablation, seven suffered regional recurrences, and five patients remained disease-free.
Conclusions
The AIM™ guidance system is an effective adjunct for laparoscopic ablation. It facilitates a high degree of accuracy and a good first-attempt success rate, and avoids the line of site interference associated with infrared systems.
Introduction
Treatment for liver cancers is often multimodal and can include systemic chemotherapy, chemoembolization, external beam and radioembolization, resection and/or ablation. For some cancers, such as early-stage hepatocellular carcinoma (HCC), ablation is reported to be just as effective as resection in terms of overall survival.1,2 Liver tumour ablations are performed percutaneously or surgically and require imaging that allows for the real-time localization of the tumour in order to ensure the accurate guidance and placement of the ablation device. Intraoperative imaging of the liver with ultrasound (US) is an essential component in the successful surgical ablation of hepatic tumours.3–5
Intraoperative US has been demonstrated to be more sensitive than preoperative imaging modalities for detecting small (<1 cm) tumours.6–9 However, a major limitation of US remains the presentation of three-dimensional space (3-D) as two-dimensional (2-D) tissue planes. This is particularly true when US is used to target tumours for which the ablation needle and the lesion must be tracked simultaneously to facilitate the accurate intersection of the needle into the tumour in order for complete ablation to be achieved. Two-dimensional US requires a high degree of skill and experience to allow accurate placement of an ablation device in 3-D space. The ability of experienced surgeons to hit a target in a single pass using US guidance varies and is reported to be as low as 59%.10
The present group has previously reported the use of 3-D guidance systems in the performance of open hepatic tumour ablations.11 The original device employed infrared (IR) tracking to simultaneously track the positions of an US probe and a microwave antenna in space. Virtual representations of these devices, along with the US image, were displayed on a stereoscopic 3-D monitor viewed by the operating surgeons. In a series of in vitro studies, this system resulted in marked increases in first pass targeting for novices and experienced surgeons, and was then trialled successfully in human open liver tumour ablations.11 However, the drawbacks of this system include the bulk of the IR tracking reflectors and the reliance on a direct, uninterrupted line of sight between the IR transmitter and the instruments. It was predicted that the flex of the US probe and ablation antenna would make targeting errors unacceptably high because the IR reflectors must be mounted on the handle of the instruments and the system must compute the position of the tip assuming that the instruments are completely rigid.
A second-generation system was therefore created using an electromagnetic tracking system in which sensors were embedded in the tips of the US and microwave ablation (MWA) antenna (Fig. 1a and b) (AIM™ Guidance System; InnerOptic Technology, Inc., Hillsborough, NC, USA) and detection performed by placing the target over a magnetic field. This removed line-of-sight limitations associated with the IR system and avoided the adding of bulk to instruments. Furthermore, it allowed the position sensors to be placed near the tips of the antenna and US probe, greatly increasing accuracy and enabling the use of flexible articulating US probes. Using in vitro and in vivo models, the present authors demonstrated that the magnetic tracking system provided better accuracy than IR tracking.10
Figure 1.
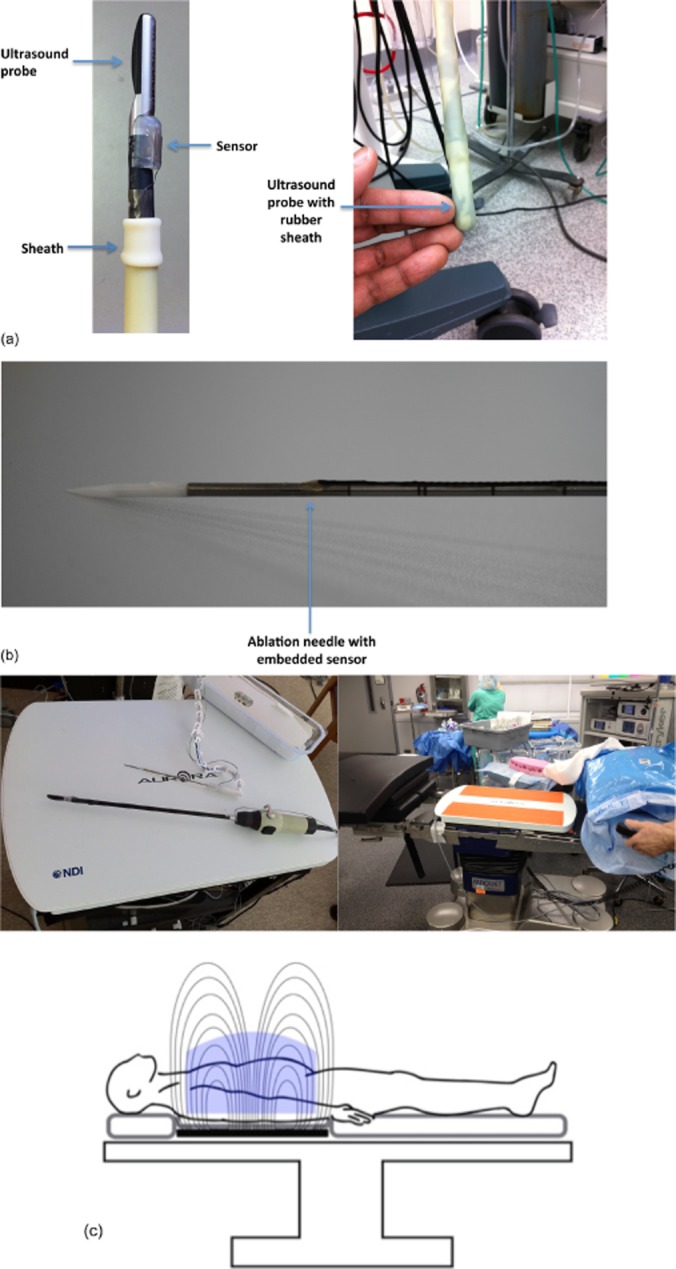
(a) A laparoscopic ultrasound probe with attached field detector. A sterile rubber sheath is placed over the probe in this design. (b) Microwave antenna with embedded sensor. (c) A magnetic field generator pad is placed beneath the patient on the operating table. Sensor coils, attached to instruments, induce electrical currents as they move through the magnetic field. These inducted currents are recorded and instrument position in three-dimensional space is calculated
The aim of this study was to evaluate the use of the InnerOptic AIM™ guidance system in laparoscopic MWA of liver tumours in a prospective human study.
Materials and methods
Institutional assurances
Approval for a human pilot study utilizing the AIM™ system for laparoscopic MWA of liver tumours was obtained from the Carolinas Medical Center Institutional Review Board. All patients would otherwise have been treated with traditional US and MWA. Informed consent was obtained from all patients.
Review of guidance system
The basic electromagnetic guidance system has been reported by the present group.10 The system (Aurora Measurement System; Northern Digital, Inc., Waterloo, ON, Canada) records the 3-D position and orientation of the ablation needle and laparoscopic US transducer using a magnetic field generator pad placed underneath the patient (Fig. 1c). Small directional field detector coils (0.3 mm in diameter, 13.0 mm in length) are installed on the instruments themselves (Fig. 1a and b). Voltages are then induced in the sensors when placed in the magnetic field; this information is transmitted to the controller and allows for the calculation of the real-time position of the instruments in space (Fig. 1c). As electromagnetic energy is readily transmitted through human tissues, the position of the device can be easily transmitted, thereby overcoming the line-of-sight issues that arose using previously tested IR systems.
Once the controller calculates the position of the devices in the field, graphical representations of the instruments (avatars) are presented on a stereoscopic display. With the aid of 3-D glasses, the operator can thus appreciate the real-time angles and positions of the instruments in three dimensions (Figs 2 and 3). The 2-D US image is simultaneously presented within this 3-D environment as a projection from the tip of the US transducer avatar and in the correct orientation to the US array. In effect, this presents the US image and the instrument locations simultaneously on the screen.
Figure 2.
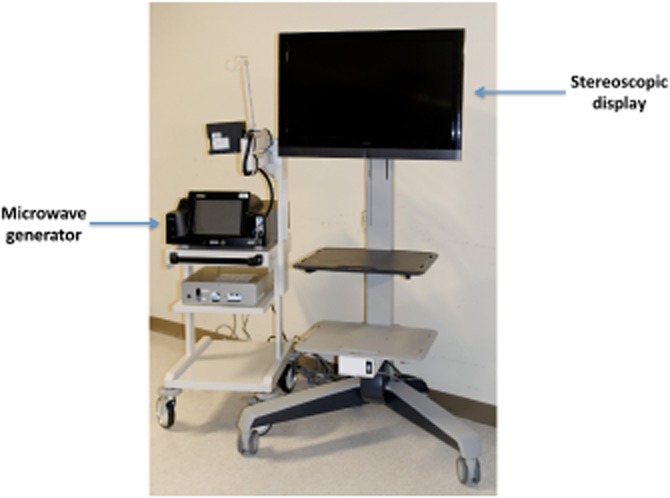
Stereoscopic display for visualizing real-time three-dimensional images
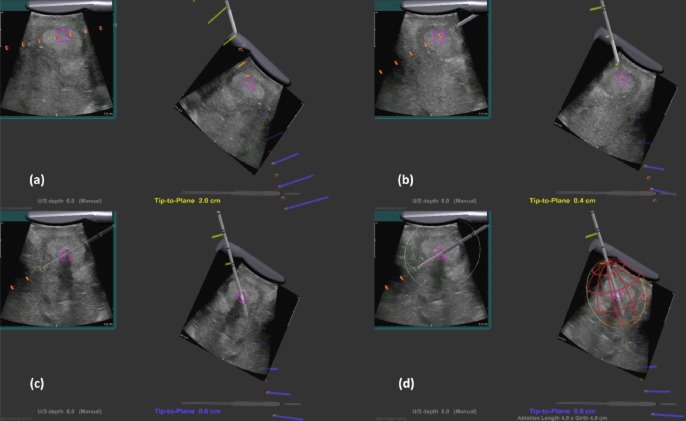
In addition to co-localizing the ablation needle and antenna with the US image, the system also projects the vector of the ablation needle beyond its physical dimensions. This allows the surgeon to project the intersection of the needle, as if it were to be advanced from its existing position, with the US image and thus to determine the angle of the needle prior to penetrating the skin. The system also predicts the point of needle intersection within the US plane and projects this point artificially onto the US image as a small purple box. Finally, once the needle has been successfully localized to the target lesion, an image of a spherical ‘cage’ can be superimposed to indicate the ablation zones predicted for different power and time settings. The spherical cage is a red–orange wire frame that is used to predict the volume of tissue to be ablated. The cage size is set by the operating surgeon based on the size of the tumour on preoperative imaging [three-phase computed tomography (CT) scan]. It represents the size of ablation the surgeon thinks is necessary to destroy the tumour completely. Thus, the surgeon can be assured that all tissue designated for destruction will be ablated and that no tissue or structures not intended for ablation (such as blood vessels) are within the field (Fig. 3).
Figure 3.
Stereoscopic three-dimensional display showing (a, b) images of the ultrasound transducer and the real-time ultrasound images, and (c) the same probe as it is positioned in 3-D space relative to the microwave antenna, shown here penetrating the tissue. (d) A red ‘cage’ graphic is superimposed to illustrate the size of the ablation cavity at the current power settings
Study design
The consent of 13 patients undergoing laparoscopic MWA of hepatic tumours was acquired prior to their enrolment in this pilot study. Targeting was performed using an InnerOptic AIM™ guidance system coupled with a ProFocus 2202 laparoscopic US scanner and transducer (B-K Medical, Inc., Peabody, MA, USA). Ablations were performed using a 1.8-mm cooled shaft antenna with a 2.45-GHz generator (Accu2i; Microsulis Medical Ltd, Denmead, UK). The primary endpoints of this Phase I trial were 30-day morbidity or mortality, local ablation success at the first postoperative imaging scan, first-time pass rate, time to lesion acquisition and time to disease progression with regional metastases. Lesion acquisition was graded on a 5-point scale (1 = very difficult, 5 = very easy).
Assessing ablation success
The completeness of ablation is quantified by two methods. The first is during the ablation time, when colour Doppler imaging is used to monitor ablation activity because there is a direct correlation between the 2-D Doppler images and the actual thermocoagulation zone in the liver tissue.12 The second method refers to the use of a postoperative triphasic CT scan performed at 4–6 weeks post-procedure to determine the completeness of ablation using the mRECIST criteria (modified response evaluation criteria in solid tumours).
Statistics
GraphPad Prism Version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) was used to complete all statistical analyses.
Results
A total of 45 ablations were performed on 34 lesions in 13 patients. The mean ± standard deviation (SD) patient age was 59.4 ± 11.1 years (range: 43–85 years). Four of the 13 patients were female (Table 1). The median number of lesions per patient was two (range: one to five lesions). The median lesion size was 17 mm (range: 5–40 mm).
Table 1.
Demographic and clinical data for the 13 patients enrolled in this study
| Patient | Age, years | Sex | Diagnosis | Lesions, n | Operative time, min | Length of stay, days | Morbidities |
|---|---|---|---|---|---|---|---|
| 1 | 43 | F | Colorectal cancer | 5 | 177 | 5 | – |
| 2 | 85 | M | HCC | 1 | 62 | 2 | – |
| 3 | 63 | F | Small bowel synovial sarcoma | 2 | 83 | 2 | – |
| 4 | 49 | M | HCC | 2 | 108 | 3 | Mild hypoxia |
| 5 | 56 | M | HCC | 2 | 118 | 4 | – |
| 6 | 52 | M | Carcinoid | 4 | 46 | 2 | – |
| 7 | 46 | M | HCC | 1 | 52 | 2 | – |
| 8 | 62 | F | HCC | 5 | 134 | 6 | Ileus, delirium, hypertension |
| 9 | 57 | M | HCC | 1 | 64 | 2 | – |
| 10 | 70 | M | Carcinoid | 5 | 96 | 3 | – |
| 11 | 63 | M | HCC | 1 | 77 | 1 | – |
| 12 | 60 | F | Lung | 3 | 113 | 8 | Hypoxia |
| 13 | 66 | M | HCC | 2 | 65 | 2 | – |
F, female; M, male; HCC, hepatocellular carcinoma.
The success rate of first-attempt passes with image guidance was 93%; three lesions required two attempts each. The mean ± SD time to lesion acquisition was 3.5 ± 2.6 min (range: 1–10 min). Ease of lesion acquisition was assessed subjectively using a 5-point scale. Of the 45 ablations, 27 were graded at 5 (very easy), four were graded at 4 (easy), four were graded at 3 (moderate), none were graded at 2 (difficult), and only one was graded at 1 (very difficult). Nine ablations were performed without guidance as they referred to surface lesions. The mean ± SD operative time was 91.9 ± 37.4 min (range: 46–177 min) and the mean ± SD hospital length of stay was 2.8 ± 1.5 days.
The mean ± SD length of follow-up was 7.8 ± 4.2 months (range: 0–16 months). One patient was lost from follow-up after discharge and thus postoperative imaging for 12 of the 13 patients was available (Fig. 4). An incomplete ablation was identified in one patient on the first postoperative scan. In a 30-mm, solitary HCC, a tongue of tumour extending centrally to the porta hepatis was not fully ablated and was apparent on postoperative imaging. The patient was treated with transarterial chemoembolization. On surveillance imaging, five patients with HCC were found not to have recurrence at an average follow-up of 6.8 months (range: 3–12 months). Two patients with HCC and all of the patients with secondary liver metastases suffered from regional recurrences during surveillance imaging.
Figure 4.
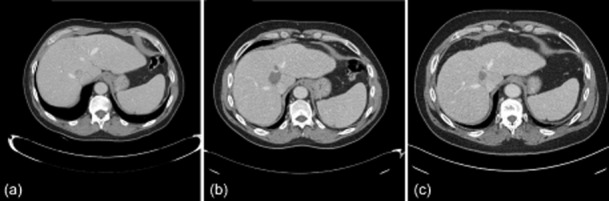
(a) Computed tomography (CT) shows a solitary hepatocellular carcinoma centrally located in the liver. (b) The first post-ablation CT demonstrates the complete ablation of the tumour. (c) This patient remains disease-free on further follow-up imaging at 14 months post-ablation
Discussion
Hepatic tumour ablation has become a mainstay in the treatment of both primary and metastatic lesions.13–15 Local recurrence attributable to incomplete thermal ablation remains a major concern despite advances in imaging, ablation system design and surgeon training. Reported incidences of incomplete hepatic ablations are typically under 5%16 and some series have reported rates as low as 2.8%,17 although others report considerably higher frequencies.18 Several factors may contribute to the reported incidence of incomplete ablations, including: the size of the series; whether the study reports primary, secondary, or primary and secondary tumours; whether the study was conducted in a single or multiple centres; the inclusion and exclusion criteria for eligibility; the type of ablation performed (radiofrequency ablation or MWA), and the experience of the surgeon.13,16,19
Even for the experienced surgeon, US targeting remains the most challenging aspect of a laparoscopic ablation. This is because, using currently available equipment, the performance of 3-D tasks using 2-D images as reference points requires the surgeon to mentally visualize the spatial relationships between the US probe, the ablation device and the lesion, a task that becomes even more difficult in laparoscopy.20,21 For both novice and experienced operators, ablations performed in the plane of the US image are more easily accomplished as the surgeon is able to see both the lesion and the tract of the needle during placement on the US. In reality, these optimal circumstances are not always possible and the placement of the ablation antenna must be achieved out of plane with the US. When this occurs, the lesion and needle do not appear on screen together until the needle passes the plane of the US. As a result, a full pass with the antenna is (usually) required before an error in targeting can be appreciated. Data from the present group using in vitro and ex vivo models report a first-time success rate of approximately 60% in expert hands. This means that to acquire a lesion out of plane for ablation (often) requires multiple full passes to accurately target the lesion, which introduces the risk for additional tissue trauma, a potential for tumour seeding and increases the time spent in the operating room.
Several commercially available computer-assisted navigational systems, developed specifically for liver and pancreas surgeons, are currently available or in development. These systems include those produced by InnerOptic Technology, Inc., Pathfinder Technology, Inc. (Nashville, TN, USA) and CAScination AG (Bern, Switzerland). These systems have many similarities and differences. All three systems continuously track the positions of the intraoperative US probe and the ablation needle and display these to the surgeon. Currently, the Pathfinder and CASination systems use optical tracking systems, which require reflectors to be attached to the handles of the needle and US probe, and cannot yet account for the flex of the US probe or needle shaft. The InnerOptic system uses an electromagnetic sensor at the tip of the needle and probe in order to account for the bending of the probe and needle. The InnerOptic and Pathfinder systems can be used laparoscopically, whereas the CASination system is currently applicable only to open procedures. CASination and Pathfinder require the user to process the preoperative CT images and to identify the boundaries of the liver and blood vessels. The surgeon must then register this preoperative plan to the patient's liver during the surgical procedure. The benefit of this is that the surgeon can see the live real-time position of the needle relative to the preoperative plan. The correspondence is not perfect because the tissue deforms between the time of the preoperative CT and the surgical procedure, and also during the surgical procedure itself as the surgeon manipulates the liver. In comparison, the InnerOptic system is simpler and faster to use (no preoperative workflow, planning or registration is required), but it does not show the locations of blood vessels or tumours beyond those apparent in the liver US images. It is important to note that all three systems are under constant and rapid development and the comparison of the three systems is subject to change with time.
Previous reports from the present group on the use of 3-D guidance for ablation in open surgery, and on the use of laparoscopic devices in simulation, showed significant increases in first-attempt success rates. For example, using a laparoscopic system in a simulated in vitro setting demonstrated a 100% first-time success rate in the hands of experts and significantly increased the accuracy of novice and intermediate users.11 The data presented herein support these findings in a prospective human trial in which a first-attempt success rate of 93% was achieved (n = 45 ablations). However, this study involved relatively few patients (n = 13) and all of the ablations were performed by surgeons who fall into the ‘expert’ category based on their prior experience and history of using MWA to treat hepatic tumours. To further demonstrate the potential of this technology it would thus be important to increase both the cohort size and the number of different surgeons using the technology. In such an investigation, it would be important to collect longterm outcomes and follow-up in order to factor in surgeon experience, the type of cases in which the technique was employed, and the underlying aggressive nature of HCC and secondary metastatic diseases of the liver.13–15 Unfortunately, this study was limited by an inability to acquire additional MWA antennae as these were custom-built prototypes (with the position sensor at the tip) and production has been halted.
In conclusion, the InnerOptic AIM™ 3-D guidance system removed the limitations imposed by line-of-sight issues that arose with an earlier IR 3-D guidance system. The InnerOptic AIM™ 3-D guidance system is seamlessly integrated for use in laparoscopy and was found to significantly improve both accuracy and first-pass success rates.
Conflicts of interest
DS, JBM and DAI are unpaid consultants and co-investigators with InnerOptic Technology, Inc. SR is an engineer and the chief technical officer at InnerOptic Technology, Inc. None of the other authors have any financial interest in the company or institution that might benefit from this publication.
References
- Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombesi P, Di Vece F, Sartori S. Resection vs thermal ablation of small hepatocellular carcinoma: what's the first choice? World J Radiol. 2013;5:1–4. doi: 10.4329/wjr.v5.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacherl J, Scheuba C, Imhof M, Zacherl M, Langle F, Pokieser P, et al. Current value of intraoperative sonography during surgery for hepatic neoplasms. World J Surg. 2002;26:550–554. doi: 10.1007/s00268-001-0266-2. [DOI] [PubMed] [Google Scholar]
- Machi J, Oishi AJ, Furumoto NL, Oishi RH. Intraoperative ultrasound. Surg Clin North Am. 2004;84:1085–1111. doi: 10.1016/j.suc.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Torzilli G, Makuuchi M. Intraoperative ultrasonography in liver cancer. Surg Oncol Clin N Am. 2003;12:91–103. doi: 10.1016/s1055-3207(02)00084-4. [DOI] [PubMed] [Google Scholar]
- Cerwenka H. Intraoperative ultrasonography during planned liver resections remains an important surgical tool. Surg Endosc. 2008;22:1137–1138. doi: 10.1007/s00464-008-9797-z. [DOI] [PubMed] [Google Scholar]
- Cervone A, Sardi A, Conaway GL. Intraoperative ultrasound (IOUS) is essential in the management of metastatic colorectal liver lesions. Am Surg. 2000;66:611–615. [PubMed] [Google Scholar]
- Machi J, Uchida S, Sumida K, Limm WM, Hundahl SA, Oishi AJ, et al. Ultrasound-guided radiofrequency thermal ablation of liver tumours: percutaneous, laparoscopic, and open surgical approaches. J Gastrointest Surg. 2001;5:477–489. doi: 10.1016/s1091-255x(01)80085-8. [DOI] [PubMed] [Google Scholar]
- Donadon M, Torzilli G. Intraoperative ultrasound in patients with hepatocellular carcinoma: from daily practice to future trends. Liver Cancer. 2013;2:16–24. doi: 10.1159/000346421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindram D, McKillop IH, Martinie JB, Iannitti DA. Novel 3-D laparoscopic magnetic ultrasound image guidance for lesion targeting. HPB. 2010;12:709–716. doi: 10.1111/j.1477-2574.2010.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindram D, Swan RZ, Lau KN, McKillop IH, Iannitti DA, Martinie JB. Real-time three-dimensional guided ultrasound targeting system for microwave ablation of liver tumours: a human pilot study. HPB. 2011;13:185–191. doi: 10.1111/j.1477-2574.2010.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd JF, Agee N, McKillop IH, Sindram D, Martinie JB, Iannitti DA. Colour Doppler ultrasonography provides real-time microwave field visualization in an ex vivo porcine model. HPB. 2011;13:400–403. doi: 10.1111/j.1477-2574.2011.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindram D, Lau KN, Martinie JB, Iannitti DA. Hepatic tumour ablation. Surg Clin North Am. 2010;90:863–876. doi: 10.1016/j.suc.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Padma S, Martinie JB, Iannitti DA. Liver tumour ablation: percutaneous and open approaches. J Surg Oncol. 2009;100:619–634. doi: 10.1002/jso.21364. [DOI] [PubMed] [Google Scholar]
- Martin RC, Scoggins CR, McMasters KM. Safety and efficacy of microwave ablation of hepatic tumours: a prospective review of a 5-year experience. Ann Surg Oncol. 2010;17:171–178. doi: 10.1245/s10434-009-0686-z. [DOI] [PubMed] [Google Scholar]
- Groeschl RT, Pilgrim CH, Hanna EM, Simo KA, Swan RZ, Sindram D, et al. Microwave ablation for hepatic malignancies: a multi-institutional analysis. Ann Surg. 2014;259:1195–1200. doi: 10.1097/SLA.0000000000000234. [DOI] [PubMed] [Google Scholar]
- Abitabile P, Maurer CA. Radiofrequency ablation of liver tumours: a novel needle perfusion technique enhances efficiency. J Surg Res. 2010;159:532–537. doi: 10.1016/j.jss.2008.08.037. [DOI] [PubMed] [Google Scholar]
- Ayav A, Germain A, Marchal F, Tierris I, Laurent V, Bazin C, et al. Radiofrequency ablation of unresectable liver tumours: factors associated with incomplete ablation or local recurrence. Am J Surg. 2010;200:435–439. doi: 10.1016/j.amjsurg.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2008;47:82–89. doi: 10.1002/hep.21933. [DOI] [PubMed] [Google Scholar]
- Xu HX, Yin XY, Lu MD, Xie XY, Xu ZF, Liu GJ. Usefulness of three-dimensional sonography in procedures of ablation for liver cancers: initial experience. J Ultrasound Med. 2003;22:1239–1247. doi: 10.7863/jum.2003.22.11.1239. [DOI] [PubMed] [Google Scholar]
- Rose SC, Hassanein TI, Easter DW, Gamagami RA, Bouvet M, Pretorius DH, et al. Value of three-dimensional US for optimizing guidance for ablating focal liver tumours. J Vasc Interv Radiol. 2001;12:507–515. doi: 10.1016/s1051-0443(07)61892-2. [DOI] [PubMed] [Google Scholar]