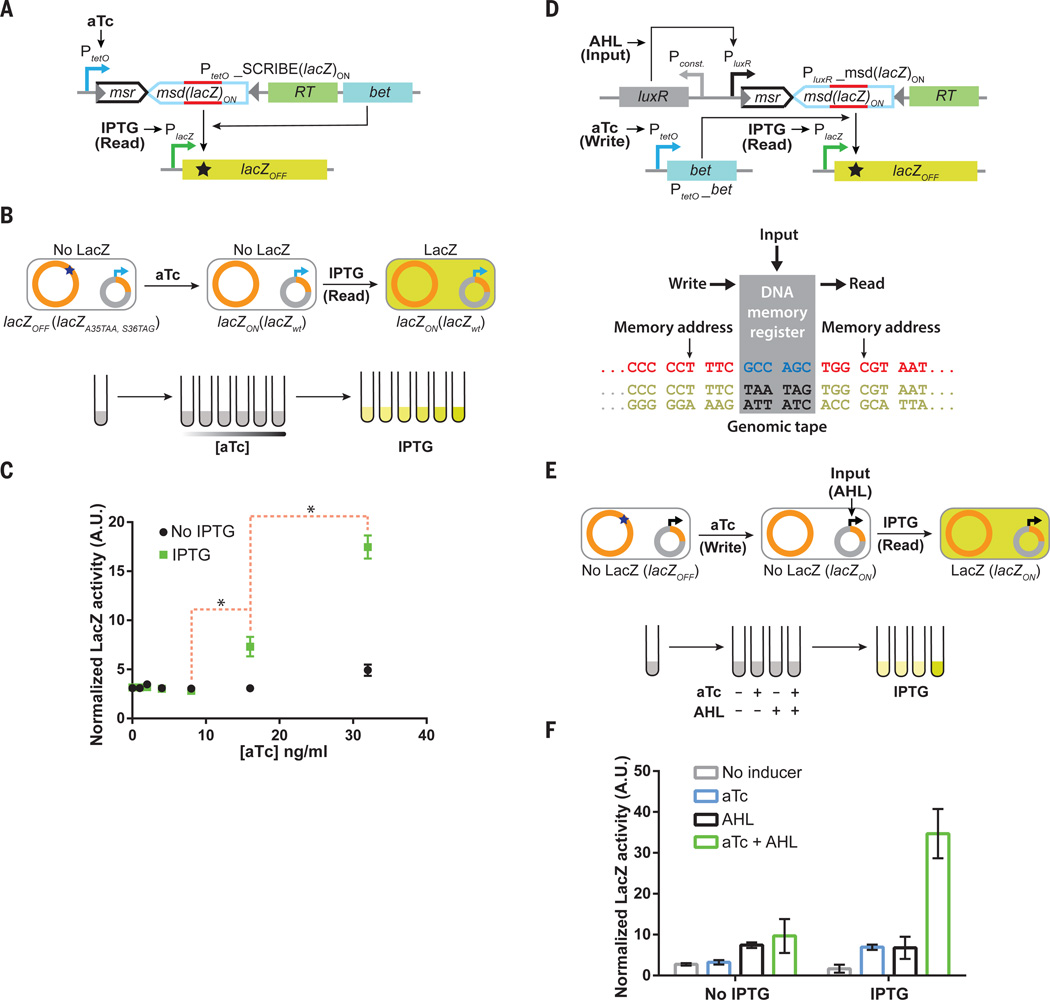
Fig. 5. SCRIBE memory operations can be decoupled into independent Input, Write, and Read operations, thus facilitating greater control over addressable memory registers in genomic tape recorders and the creation of sample-and-hold circuits.
(A) We built a circuit where information about the first inducer (aTc) is recorded in the population, which can then be read later upon addition of a second inducer (IPTG) that triggers a “Read” operation. We created an IPTG-inducible lacZOFF locus in the DH5αPRO background, which contains the full-length lacZ gene with two premature stop codons inside the open-reading frame. Expression of ssDNA(lacZ)ON from the aTc-inducible SCRIBE(lacZ)ON cassette results in the reversion of the stop codons inside lacZOFF to yield the lacZON genotype. (B) Cells harboring the circuit shown in A) were grown in the presence of different levels of aTc for 24 h at 30°C to enable recording into genomic DNA. Subsequently, cell populations were diluted into fresh media without or with IPTG (1 mM) and incubated at 37°C for 8 hours. (C) Total LacZ activity in these cultures was measured using a fluorogenic lacZ substrate (FDG) assay. The red dashed brackets marked with asterisks connect the closest data points of IPTG-induced samples that are statistically significant (p-value < 0.05 based on one-tailed Welch’s t-test). (D) We extended the circuit in A) to create a sample-and-hold circuit where “Input”, “Write”, and “Read” operations are independently controlled. This feature enables the creation of addressable Read/Write memory registers in the genomic DNA tape. Induction of cells with the “Input” signal (AHL) produces ssDNA(lacZ)ON, which targets the genomic lacZOFF locus for reversion to the wild-type sequence. In the presence of the “Write” signal (aTc), which expresses Beta, ssDNA(lacZ)ON is recombined into the lacZOFF locus and produces the lacZON genotype. Thus, the “Write” signal enables the “Input” signal to be sampled and held in memory. The total LacZ activity in the cell populations is retrieved by adding the “Read” signal (IPTG). (E) Cells harboring the circuit shown in D) were induced with different combinations of aTc (100 ng/ml) and AHL (50 ng/ml) for 24 h, after which the cultures were diluted in fresh media with or without IPTG (1 mM). These cultures were then incubated at 37°C for 8 hours and assayed for total LacZ activity with the FDG assay. (F) Cell populations that received both the “Input” and “Write” signals, followed by the “Read” signal exhibited enhanced levels of total LacZ activity. Error bars indicate the standard error of the mean for three independent biological replicates.