Abstract
Background
Atrial pacing is indicated for sinus node dysfunction (SND) after Fontan surgery; preferred lead implantation technique is debated. We compare outcomes of transvenous (TV) and epicardial (Epi) atrial lead implants in this population.
Methods
Retrospective review of Fontan patients undergoing atrial lead implant between 1992 and 2007. Demographics, lead performance data, and outcomes were analyzed.
Results
78 patients had 90 leads implanted: 25 via TV route and 65 via Epi route. Median follow-up was 1.6 years (TV) and 3.6 years (Epi). TV leads were implanted in older patients (23.1 vs 9.3 years, P < 0.001) and at longer intervals after Fontan (15.2 vs 4.9 years, P < 0.001). Pacing indication for most TV leads was SND, while Epi leads were also indicated for atrioventricular block. Acute complication rates were similar (8% TV vs 19% Epi, P = 0.23), but median hospital stay was shorter for TV (2 vs 5 days, P = 0.03). Thrombus was observed in five patients (two in TV; three in Epi), but no thromboembolic events were observed. Mean lead survival was similar (TV 9.9 vs Epi 7.8 years, P = NS). Energy threshold was lower at implant for TV leads (0.9 vs 2.2 μJ, P = 0.049), but similar at follow-up (1.2 vs 2.6 μJ, P = 0.35). Atrial sensing was unchanged over time for TV (2.2 to 2.1 mV, P = NS), but decreased for Epi (3.3 to 2.5 mV, P = 0.02).
Conclusions
Compared to epicardial leads, transvenous atrial pacing leads may be placed in Fontan patients with lower procedural morbidity and equivalent expectation of lead performance and longevity. (PACE 2009; 32:779–785)
Keywords: congenital heart disease, arrhythmia, fontan procedure, permanent pacing
Introduction
Palliative surgery for a functional single ventricle culminates in a Fontan-type operation. Although many surgical modifications have improved the hemodynamic status of the Fontan circulation, postoperative rhythm disturbances are prevalent, particularly sinus node dysfunction (SND). Because loss of sinus rhythm may result in symptoms associated with low cardiac output and/or promote the occurrence of atrial tachyarrhythmias, permanent atrial pacemakers may be indicated in selected patients.1–4
Pacemaker implant is the most frequently required cardiac surgical procedure after Fontan-type operations,5 and we have previously noted that pacemakers were implanted in 9.2% of Fontan patients.6 In the absence of higher-degree atrioventricular (AV) block, there are practically two therapeutic options for pacemaker therapy of SND following the Fontan operation: epicardial lead placement (which also allows for dual-chamber pacing) or transvenous atrial AAI (R) pacing.
Epicardial lead placement has been most commonly used for permanent atrial pacing in Fontan patients with SND, but advances in lead and pacemaker technology now offer further therapeutic and surgical opportunities, and transvenous leads have also been utilized. Each approach has distinct concerns and constraints. Epicardial lead placement requires more invasive atrial exposure (i.e., sternotomy or thoracotomy) and the associated procedural risk is a significant disadvantage (although frequen need for reoperation for other indications mitigates this somewhat, as lead placement can be performed at the same procedure (i.e., “piggybacked”).7 Chronic epicardial lead performance has historically been inferior, with higher pacing and lower sensing thresholds and earlier battery depletion rates than transvenous systems.8 Higher lead failure rates are also noted, with one report observing a 40% failure rate of epicardial atrial leads within 5 years of implantation.1
Avoiding these problems by using a transvenous approach is attractive but may be offset by limitations or concerns with this approach. These include: (1) possible Fontan pathway obstruction by transvenous leads; (2) potential increased risk of thromboembolism, especially in patients with residual intracardiac right-to-left shunting,9 (3) anatomical constraints limiting feasibility of ventricular pacing following most Fontan-type surgical repairs; and (4) the use of certain Fontan approaches that completely exclude vascular access to atrial tissues (i.e., the prosthetic, extracardiac conduit type).
There are contradictory results on the outcome and performance of pacing lead implant in either epicardial (Epi)10,11 or transvenous (TV)12,13 leads. Although some comparison studies of epicardial and transvenous lead survival in coronary heart disease or pediatric patients have been performed,14–19 there is no study comparing epicardial and transvenous atrial leads electively placed in patients following Fontan operation without other concomitant surgical indication. In this study, we sought to test the hypothesis that epicardial and transvenous atrial leads in patients following Fontan operation had equivalent procedural outcomes and lead performance.
Material and Methods
Data Collection
Retrospective review was approved by the Institutional Review Board. The study group was identified from the medical records of all patients who had already had a Fontan procedure, and who underwent permanent atrial lead implantation without any other concomitant surgery at Children’s Hospital Boston, between January 1992 and March 2007. Pacing and sensing thresholds were obtained at implantation and at follow-up. For analysis, patients were subcategorized according to the pacing lead implant route into two groups: TV versus Epi route.
Definitions
Lead Failure
Lead failure was defined by the need for replacement or abandonment due to loss of capture and/or sensing, lead displacement, conductor fracture, insulation break, or exit block.
Threshold, Impedance, Energy Thresholds (ET)
Pacing and sensing thresholds were determined via pacemaker system analyzers. The thresholds were determined by decreasing the voltage at a fixed pulse width (typically 0.5 ms) until there was failure to capture, with threshold defined as the lowest voltage at which there was consistent capture.20
ET
ET was defined as the least amount of energy producing consistent capture outside the refractory period, according to the following formula:
Outcome Variables
The primary outcome focused on mortality and morbidity, both early (<30 days postimplant) and late. Secondary outcomes analyzed included lead failure and lead functional parameters at the time of implant and follow-up. Outcome variables were compared between the two lead groups.
Statistical Analysis
Continuous variables are summarized as mean ± standard deviation or median and interquartile range (25th, 75th percentile). Categorical variables are represented by frequencies and percentages. All patient characteristics are compared between the two groups: t-test for continuous variables that satisfied the normality assumption, Wilcoxon rank sum test for data that deviates from normality, and ×2 test or Fisher’s exact test for comparison of frequency distribution of categorical variables. Freedom from lead failure was plotted using Kaplan-Meier curves, with comparisons by log-rank tests. Predictors were explored in univariate and multivariate logistic regression analyses from which odds ratios and 95% confidence intervals were generated. Variables significant at the 0.2 level in univariate analyses were included in a stepwise multivariate logistic regression model. Two-tailed P-values < 0.05 were considered statistically significant. Analyses were performed with StatView J-5.0 PPC (SAS Institute Inc., Cary, NC, USA).
Results
Patient Characteristics
Clinical characteristics of the study population are shown in Table I. Seventy-eight post-Fontan patients undergoing atrial lead implant were identified and reviewed. The routes of atrial lead implant in this population were transvenous in 25, epicardial (sternotomy, n = 50; or thoracotomy, n = 15) in 65. The choice of either transvenous or surgical approach was dependent on the joint decision of cardiologist and surgeon. Underlying diagnoses of single ventricle physiology included tricuspid atresia (n = 24), double-inlet left ventricle/single left ventricle (n = 18), double outlet right ventricle (n = 12), hypoplastic left heart syndrome (n = 10), and others (n = 14). Median age at Fontan operation was 8.7 years (2.8, 15.9 years) for TV and 3.5 years (2.5, 8.1) for Epi (p = n.s). TV leads were implanted in older patients (median age: 24.2 vs 9.5 years, P < 0.001), at longer interval after Fontan surgery (median: 15.2 vs 4.9 years, P< 0.001). Median follow-up duration was 1.6 years (1.0, 4.4 years) for TV and 3.6 years (1.9, 6.2 years) for Epi. Indication for TV was more likely to be SND, while Epi pacing was also indicated for AV block. Pacing modes were exclusively AAI/AAI-T in TV and mostly DDD in Epi leads (Table I). Pacemaker leads used are listed in Table II. All 23 TV patients (warfarin, n = 17; aspirin, n = 6) and 42/56 Epi patients (75%; warfarin, n = 18; aspirin, n = 24) were anticoagulated.
Table 1.
Baseline Characteristics and Pacemaker Considerations
| Transvenous | Epicardial | P-value | |
|---|---|---|---|
| Age at implant, years | 24.2 (2, 1) | 9.5 (2, 1) | P <0.001 |
| Age at Fontan, years | 8.7 (2.8, 15.9) | 3.5 (2.5, 8.1) | n.s |
| Interval Fontan Implant, years | 15.2 (2, 1) | 4.9 (2, 1) | P <0.001 |
| Type of Fontan | P <0.001 | ||
| ARC | 14 | 13 | |
| LT | 7 | 52 | |
| Fenestration | 5 | 49 | |
| Hospital stay, days | 3.6 | 5.9 | P <0.001 |
| Follow-up years | 3.3 | 4.4 | n.s |
| Indication | |||
| SND | 80% | 58% | |
| AV block | 0% | 32% | |
| AFL | 20% | 10% | |
| Pacemaker mode | |||
| AAI/AAI-T | 96% | 38% | |
| ODD | 4% | 62% | |
| Lead characteristics | |||
| Steroid eluting | 19 (83%) | 35 (68%) | n.s |
| Polarity; bipolar | 23 (100%) | 45 (88%) | n.s |
| Fixation; screw-in | 22 (100%) | 19 (37%) | <0.001 |
APC = atrial to pulmonary artery connection; LT = lateral tunnel; SND = sinus node dysfunction; AV = atrioventricular; AFL = atrial flutter.
*Non-normalty distributed continuous variables are expressed as median (25th, 75th percentile).
Table II.
Lead Characteristics
| Steroid | Fixation | Polarity | N | ||||
|---|---|---|---|---|---|---|---|
| Medtronic | 4068* | Capsure Fix | SE | Screw in | B | Endocardial | 5 |
| 5076 | Capsurs Fix Novus | SE | Screw in | Bi | Endocardial | 2 | |
| 4965 | Capsure Epi | SE | Suture on | Uni | Epicardial | 2 | |
| 4968 | Capsure Epi | SE | Suture on | Bi | Epicardial | 27 | |
| 5071 | IS-1 sutureless | Non | Screw in | Uni | Epicardial | 2 | |
| 5815A | Non | Suture on | Uni | Epicardial | 2 | ||
| Guidant/CPI | 4269* | Sweet tip | Non | Screw in | Bi | Endocardial | 15 |
| 4316 | Sutureless | Non | Suture on | Bi | Epicardial | 1 | |
| 4469 | Fineline | SE | Screw in | Bi | Endocardial | 6 | |
| Pacesetter/SJM | 1388T* | Tendril DX | SE | Screw in | Bi | Endocardial | 1 |
| 1488T | Tendril | SE | Screw in | Bi | Endocardial | 2 | |
| 1688T | Tendril SDX | SE | Screw in | Bi | Endocardial | 8 | |
| Others/unknown | 22 |
Leads designed for endocardial use but placed epicardially.
Mortality and Morbidity
There was no early mortality in either epicardial or endocardial pacing groups. Two late deaths occurred in the Epi group, but the causes were not related to pacemaker lead function. Acute complication occurred in 2/25 8%) of TV (pneumothorax n = 1, skin erosion n = 1) and 12/65 (19%, P = 0.23) of Epi implants (effusions n = 5, heart failure n = 1, retained foreign body n = 1, pneumothorax n = 1, hematoma n = 1, disconnection n = 1, sepsis n = 1, and blood loss n = 1). Median hospital stay was shorter in TV (2.0 vs 4.5 days, P = 0.03). Thoracotomy was a univariate predictor of acute complication (heart rate 6.00, P = 0.01). At follow-up, pocket infection occurred in one Epi patient. Thrombus formation was confirmed by echocardiography in three patients (one TV, two Epi) and deemed possible but unconfirmed in two more (one TV, one Epi), all despite use of anticoagulation therapy. Of the five patients developing or possibly developing thrombus, one was in the TV group and four in Epi. Three out of the five had been on warfarin therapy at the time of diagnosis (one TV, two Epi). Four of the five thrombi were identified in right atrium, and one in left ventricle. No clinical thromboembolic event was observed in either group.
Pacemaker Parameters: Sensing Threshold/Lead Impedance/ET
Sensing Threshold
The median acute sensing threshold of endocardial atrial leads at implantation was 1.8 (1.2, 2.6) mV compared with 3.5 (2.0, 4.2) mV for epicardial leads (P = 0.006). At follow-up, the median sensing threshold for the endocardial group was 1.6 (1.2, 2.5) mV compared to 2.6 (1.4, 3.1) mV for the epicardial group (P = n.s). Atrial sensing was unchanged over time for TV (1.8 to 1.6 mV, P = n.s), but significantly decreased in Epi (3.5 to 2.6 mV, P = 0.006) (Fig. 1).
Figure 1.
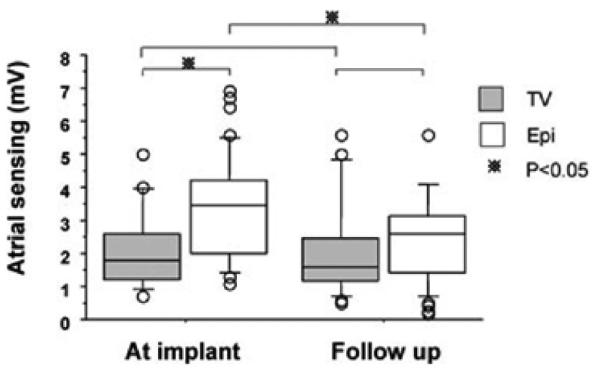
Kaplan-Meier curve plots free from atrial lead failure between TV lead implant versus epicardial lead inplant. Sesed atrial amplitude. * indicates P < 0.05.
Lead Impedance
The mean lead impedance for the atrial endocardial leads was 530 (500, 601) Ω compared to 680 (539, 831) Ω for the epicardial group. At follow-up, the mean lead impedance was 540 (469, 716) Ω and 640 (557, 761) Ω for TV and Epi groups, respectively (P < 0.05) (Fig. 2).
Figure 2.
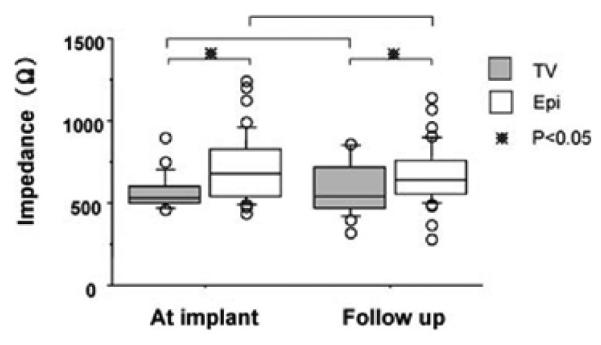
Lead impedance. * indicates P < 0.05.
ET
The mean ET was lower at implant for TV leads (0.9 vs 2.2 μJ, P = 0.049), but similar for both leads on follow-up (1.2 vs 2.6 μJ, P = 0.35) (Fig. 3).
Figure 3.
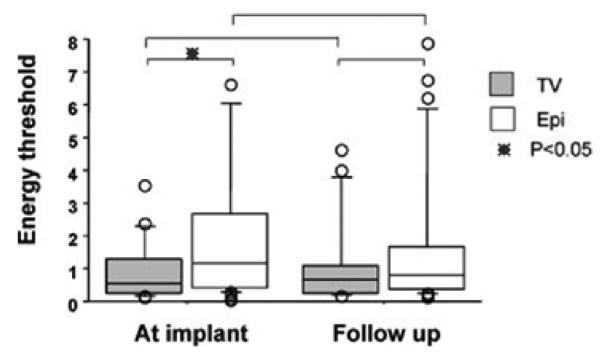
Energy treshold. * indicates P < 0.05.
Lead Failure
Three TV leads failed in two patients (9%), while 12 Epi leads failed in 10 patients (18%). However, due to difference in follow-up duration, mean duration of freedom from lead failure was not significantly different (TV 9.9 vs Epi 8.0 years, P = n.s). Thus, route of lead implant was not predictive of lead failure. Univariate predictors for lead failure were initial high ET (HR 1.62, P = 0.006) and nonsteroid eluting lead (HR 14.0, P =0.002) (Fig. 4).
Figure 4.
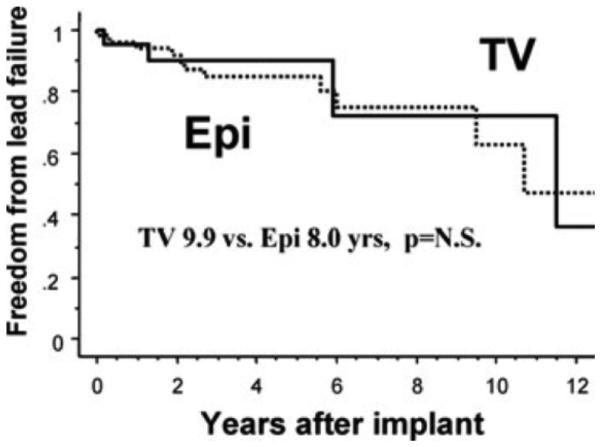
Freedom from lead failure.
Discussion
The present retrospective study compared epicardial and transvenous atrial pacemaker leads in Fontan patients. Atrial lead implant is often indicated in these patients, and the outcomes of this procedure performed without other concomitant surgery have not been previously reported in this group. The data show that transvenous atrial pacing leads have been implanted in these patients with lower procedural morbidity than epicardial leads, and have had an equivalent expectation of lead performance and longevity.
Morbidity
In the current study, Epi subgroup had more frequent acute complications and a longer hospital stay. Univariate analysis revealed that thoracotomy was a predictor for morbidity, especially risk of pleural effusion, which is concordant to the previous reports.1,8 Multivariate analysis did not show that route of lead implant was predictive of morbidity in our study.
The Fontan operation increases the risk of coagulopathy such as thromboembolism, particularly systemic venous thrombosis in the presence of the residual right-to-left shunting, but the use of long-term anticoagulation remains controversial in Fontan patients and there is no evidence based consensus with regard to type or duration of prophylactic therapy. Given the small number of patients in this study, one can only state that the prevalence of silent thrombosis serendipitously observed in our group of atrial-paced Fontan patients was ~6% over the follow-up period (95% binomial confidence intervals: 2% – 14%) and the rate of clinical thromboembolic events is likely to be <4% (observed rate: 0%, 95% binominal confidence intervals: 0%–4%). These data support the perception of thrombotic risk in these patients, although thrombosis is not specifically associated with the use of TV leads. It is the practice in this institution to start prophylactic warfarin therapy after placement of a TV atrial pacing lead, and many patients with Epi leads are anticoagulated for additional indications. Moreover, in addition to warfarin therapy, preimplant device closure of atrial level right-to-left shunts may be prudent prior to transvenous placement of the atrial lead.
Pacing and Sensing Characteristics
In the present study, sensed atrial amplitude of TV at implant was lower than that of Epi, a finding that is discordant to results of the previous studies in the general population with congenital heart disease.17,19 Possible reasons for this may include operator learning curve for TV placement, differences between epicardial and endocardial properties of the atrial myocardium, and intrinsic, biasing differences in the subpopulations selected for TV versus Epi in the post-Fontan groups.21,22 Another reason may be the possibility of lead placement on the superior and leftsided atrial tissue in some of the Epi subgroup, depending on the surgical approach and the atrial exposure.23 ET of pacing at implant was significantly lower in TV subgroup than Epi subgroup, but lead impedance throughout the follow-up was also lower in TV subgroup. It is not clear in the current study what impact these data have on the longevity of the pacemaker generator.
In our previous cohort of Fontan patients, Fishberger et al. found that AV synchrony with dual-chamber pacing probably conferred longterm survival benefit compared to single-chamber ventricular pacing, but this did not reach statistical significance.6 Several reports indicate that post-Fontan patients with bradycardia, especially associated with ventricular dysfunction, clearly benefit from the physiological pacing.18,24,25 In this setting, atrial pacing is sufficient, and in some centers, prophylactic atrial pacing lead placement is performed during the Fontan operation or the TCPC conversion, which mitigates any added operative risk associated with atrial pacing.8 However, when the decision for atrial pacing is made after the Fontan procedure is complete, it is reasonable to weigh the risks of a sternotomy or thoracotomy needed to perform epicardial lead placement against those associated with transvenous placement of an atrial pacing, as described above. In such setting, single-chamber transvenous atrial pacing is not obsolete, but an acceptable and realistic option in post Fontan patients with SND.
Certain absolute or relative contraindications to atrial endocardial pacing may be present in some patients. First, some are excluded by extracardiac-type TCPC, which precludes the transvenous approach to lead insertion. Second, although it is unclear whether TV lead placement constitutes an additive risk of thrombosis in these patients, right-to-left atrial level shunting that cannot be closed for anatomical or physiological reasons without surgery are probably better served by epicardial lead placement. Finally, dual-chamber pacing is necessary for those with, and may be elected for those at high risk for AV block (e.g., polysplenia, AV discordance). Although this may be feasible though transvenous placement of a ventricular lead via the coronary sinus, epicardial ventricular lead placement is much more commonly used.
We have concluded that TV atrial leads function at an equivalent level as Epi leads in post-Fontan patients without intracardiac shunting, and may be associated with shorter postoperative length of stay and fewer complications associated with the implant procedure.
Study Limitations
The retrospective and observational study design limits the scope of data collection to clinically-indicated follow-up and the occurrence of significant clinical events. Thus, the occurrence of thrombosis and vascular occlusion, which include periods of occult or “preclinical” evolution, may be underestimated due to lack of symptoms or signs. Although the thromboembolic event rate appears low in anticoagulated patients with both lead types, this type of retrospective study (which did not include routine angiography or echocardiography) cannot address this important issue.
Our assessment of lead equivalency may be somewhat biased, given the lead technologies utilized in the present study. A somewhat larger fraction of transvenous leads implanted were steroid eluting and bipolar, which are known to improve the stimulation threshold in an active-fixation atrial permanent pacing lead.26,27
Conclusions
When feasible and in the absence of intraatrial shunting, transvenous atrial pacing leads may be placed in Fontan patients with lower procedural morbidity than epicardial leads, and equivalent expectation of lead performance and longevity. The thromboembolic event rate appears low in anticoagulated patients with both lead types, but the study as designed cannot address this important issue.
Acknowledgments
Funding: This work was supported by the National Institutes of Health under award number: T32HL007572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest: Triedman – Speaker’s honorarium, Boston Scientific; Cecchin – Speaker’s honorarium, St. Jude Medical.
References
- 1.Cohen MI, Wernovsky G, Vetter VL, Wieand TS, Gaynor JW, Jacobs ML, Spray TL, et al. Sinus node dysfunction after a systematically staged Fontan procedure. Circulation. 1998;98:352II–358II. [PubMed] [Google Scholar]
- 2.Cohen MI, Bridges ND, Gaynor JW, Hoffman TM, Wernovsky G, Vetter VL, Spray TL, et al. Modifications to the cavopulmonary anastomosis do not eliminate early sinus node dysfunction. J Thorac Cardiovasc Surg. 2000;120:891–901. doi: 10.1067/mtc.2000.109708. [DOI] [PubMed] [Google Scholar]
- 3.Bae E-J, Lee J-Y, Noh C-I, Kim W-H, Kim Y-J. Sinus node dysfunction after Fontan modifications influence of surgical method. Int J Cardiol. 2003;88:285–291. doi: 10.1016/s0167-5273(02)00530-2. [DOI] [PubMed] [Google Scholar]
- 4.Driscoll DJ, Offord KP, Feldt RH, Schaff HV, Puga FJ, Danielson GK. Five-to fifteen-year follow-up after Fontan operation. Circulation. 1992;85:469–496. doi: 10.1161/01.cir.85.2.469. [DOI] [PubMed] [Google Scholar]
- 5.Petko M, Myung RJ, Wernovsky G, Cohen MI, Rychik J, Nicolson SC, Spray TL, et al. Surgical reinterventions following the Fontan procedure. Eur J Cardiothorac Surg. 2003;24:255–259. doi: 10.1016/s1010-7940(03)00257-4. [DOI] [PubMed] [Google Scholar]
- 6.Fishberger SB, Wernovsky G, Gentles TL, Gamble WJ, Gauvreau K, Burnett J, Mayer JE, et al. Long-term outcome in patients with pacemakers following the Fontan operation. Am J Cardiol. 1996;77:887–889. doi: 10.1016/s0002-9149(97)89191-6. [DOI] [PubMed] [Google Scholar]
- 7.Sachweh JS, Vazquez-Jimenez JF, Schondube FA, Daebritz SH, Dörge H, Muühler EG, Messmer BJ. Twenty years experience with pediatric pacing: Epicardial and transvenous stimulation. Eur J Cardiothorac Surg. 2000;17:455–461. doi: 10.1016/s1010-7940(00)00364-x. [DOI] [PubMed] [Google Scholar]
- 8.Heinemann MK, Gass M, Breuer J, Ziemer G. DDD pacemaker implantation after Fontan-type operations. Pacing Clin Electrophysiol. 2003;26:492–495. doi: 10.1046/j.1460-9592.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 9.Khairy P, Landzberg MJ, Gatzoulis MA, Mercier LA, Fernandes SM, Côté JM, Lavoie JP, et al. Epicardial Versus Endocardial Pacing and Thromboembolic Events Investigators. Transvenous pacing leads and systemic thromboemboli in patients with intracardiac shunts: A multicenter study. Circulation. 2006;113:2391–2397. doi: 10.1161/CIRCULATIONAHA.106.622076. [DOI] [PubMed] [Google Scholar]
- 10.Dodge-Khatami A, Rahn M, Prétre R, Bauersfeld U. Dual chamber epicardial pacing for the failing atriopulmonary Fontan patient. Ann Thorac Surg. 2005;80:1440–1444. doi: 10.1016/j.athoracsur.2005.03.128. [DOI] [PubMed] [Google Scholar]
- 11.Cohen MI, Vetter VL, Wernovsky G, Bush DM, Gaynor JW, Iyer VR, Spray TL, et al. Epicardial pacemaker implantation and follow-up in patients with a single ventricle after the Fontan operation. J Thorac Cardiovasc Surg. 2001;121:804–811. doi: 10.1067/mtc.2001.113027. [DOI] [PubMed] [Google Scholar]
- 12.Shah MJ, Nehgme R, Carboni M, Murphy JD. Endocardial atrial pacing lead implantation and midterm follow-up in young patients with sinus node dysfunction after the Fontan procedure. Pacing Clin Electrophysiol. 2004;27:949–954. doi: 10.1111/j.1540-8159.2004.00564.x. [DOI] [PubMed] [Google Scholar]
- 13.Hansky B, Blanz U, Peuster M, Gueldner H, Sandica E, Crespo-Martinez E, Mathies W, et al. Endocardial pacing after Fontan-type procedures. Pacing Clin Electrophysiol. 2005;28:140–148. doi: 10.1111/j.1540-8159.2005.04006.x. [DOI] [PubMed] [Google Scholar]
- 14.Walker F, Siu S, Woods S, Cameron DA, Webb GD, Harris L. Long-term outcomes of cardiac pacing in adults with congenital heart disease. J Am Coll Cardiol. 2004;43:1894–1901. doi: 10.1016/j.jacc.2003.12.044. [DOI] [PubMed] [Google Scholar]
- 15.Fortescue EB, Berul CI, Cecchin F, Walsh EP, Triedman JK, Alexander ME. Comparison of modern steroid-eluting epicardial and thin transvenous pacemaker leads in pediatric and congenital heart disease patients. J Interv Card Electrophysiol. 2005;14:27–36. doi: 10.1007/s10840-005-3797-x. [DOI] [PubMed] [Google Scholar]
- 16.Fortescue EB, Berul CI, Cecchin F, Walsh EP, Triedman JK, Alexander ME. Patient, procedural, and hardware factors associated with pacemaker lead failures in pediatrics and congenital heart disease. Heart Rhythm. 2004;1:150–159. doi: 10.1016/j.hrthm.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 17.Beaufort-Krol GC, Mulder H, Nagelkerke D, Waterbolk TW, Bink-Boelkens MT. Comparison of longevity, pacing, and sensing characteristics of steroid-eluting epicardial versus conventional endocardial pacing leads in children. J Thorac Cardiovasc Surg. 1999;17:523–528. doi: 10.1016/s0022-5223(99)70332-6. [DOI] [PubMed] [Google Scholar]
- 18.Silvetti MS, Drago F, Grutter G, De Santis A, Di Ciommo V, Ravá L. Twenty years of paediatric cardiac pacing: 515 pacemakers and 480 leads implanted in 292 patients. Europace. 2006;8:530–536. doi: 10.1093/europace/eul062. [DOI] [PubMed] [Google Scholar]
- 19.Odim J, Suckow B, Saedi B, Laks H, Shannon K. Equivalent performance of epicardial versus endocardial permanent pacing in children: A single institution and manufacturer experience. Ann Thorac Surg. 2008;85:1412–1416. doi: 10.1016/j.athoracsur.2007.12.075. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton RM, Chiu C, Gow RM, Williams WG. A comparison of two stab-on unipolar epicardial pacing leads in children. Pacing Clin Electrophysiol. 1997;20:631–636. doi: 10.1111/j.1540-8159.1997.tb03881.x. [DOI] [PubMed] [Google Scholar]
- 21.de Groot NM, Kuijper AF, Blom NA, Bootsma M, Schalij MJ. Three-dimensional distribution of bipolar atrial electrogram voltages in patients with congenital heart disease. Pacing Clin Electrophysiol. 2001;24:1334–1342. doi: 10.1046/j.1460-9592.2001.01334.x. [DOI] [PubMed] [Google Scholar]
- 22.de Groot NM, Schalij MJ, Zeppenfeld K, Blom NA, Van der Velde ET, Van Der Wall EE. Voltage and activation mapping: How the recording technique affects the outcome of catheter ablation procedures in patients with congenital heart disease. Circulation. 2003;108:2099–2106. doi: 10.1161/01.CIR.0000092893.11893.38. [DOI] [PubMed] [Google Scholar]
- 23.Ramesh V, Gaynor JW, Shah MJ, Wieand TS, Spray TL, Vetter VL, Rhodes LA. Comparison of left and right atrial epicardial pacing in patients with congenital heart disease. Ann Thorac Surg. 1999;68:2314–2319. doi: 10.1016/s0003-4975(99)01053-x. [DOI] [PubMed] [Google Scholar]
- 24.Barber BJ, Batra AS, Burch GH, Shen I, Ungerleider RM, Brown JW, Turrentine MW, et al. Acute hemodynamic effects of pacing in patients with Fontan physiology: A prospective study. J Am Coll Cardiol. 2005;46:1937–1942. doi: 10.1016/j.jacc.2005.07.045. 2288. [DOI] [PubMed] [Google Scholar]
- 25.Barber G, Di Sessa T, Child JS, Perloff JK, Laks H, George BL, Williams RG. Hemodynamic responses to isolated increments in heart rate by atrial pacing after a Fontan procedure. Am Heart J. 1988;115:837–841. doi: 10.1016/0002-8703(88)90887-3. [DOI] [PubMed] [Google Scholar]
- 26.Crossley GH, Brinker JA, Reynolds D, Spencer W, Johnson WB, Hurd H, Tonder L, et al. Steroid elution improves the stimulation threshold in an active-fixation atrial permanent pacing lead. A randomized,controlled study. Model 4068 Investigators. Circulation. 1995;92:2935–2939. doi: 10.1161/01.cir.92.10.2935. [DOI] [PubMed] [Google Scholar]
- 27.Tomaske M, Gerritse B, Kretzers L, Pretre R, Dodge-Khatami A, Rahn M, Bauersfeld U. A 12-year experience of bipolar steroid-eluting epicardial pacing leads in children. Ann Thorac Surg. 2008;85:1704–1711. doi: 10.1016/j.athoracsur.2008.02.016. [DOI] [PubMed] [Google Scholar]


